Keywords
Non-AIDS defining cancers, cohort studies, coinfection, hepatitis
Non-AIDS defining cancers, cohort studies, coinfection, hepatitis
In high-income countries, life expectancy of people living with HIV (PLWHIV) on combination antiretroviral therapy (cART) is approaching that of the general population1. For example, in Switzerland 2006 to 2013, PLWHIV with higher education had an estimated life expectancy similar to individuals from the general population with compulsory education2. Similarly, in Canada 2008 to 2012, life expectancy at age 20 years in HIV-positive people was 89% of the life expectancy in the general population3. In line with the increasing life expectancy, morbidity and mortality of PLWHIV on cART are no longer dominated by AIDS-defining events, but also affected by cardiovascular events and non-AIDS defining malignancies (NADM)1–5.
An analysis of causes of death in the Data collection on Adverse events of Anti-HIV Drugs (a collaboration of eleven HIV cohort studies from Europe, the USA and Australia), found that mortality decreased from 1999 to 2011 for most causes of death, including AIDS-related, liver-related, cardiovascular and other or unknown causes4. However, in this collaborative study4 and in other cohorts5–7, mortality from NADM remained stable over time. This type of cancer is now the most common cause of non-AIDS deaths in PLWHIV in high-income countries8. Furthermore, compared to the general population, the incidence of NADM is increased in PLWHIV, with a standardized incidence ratio for all NADM combined ranging from 1.6 to 2.8 across different studies9.
Several factors contribute to the higher risk of NADM among HIV-positive individuals, including prolonged exposure to immunodeficiency, a higher prevalence of smoking, and infections with oncogenic viruses such as human papilloma virus (HPV) or hepatitis B (HBV) or hepatitis C (HCV) viruses10–12. Direct pro-oncogenic effects of HIV, inflammation, and coagulation pathways may also play a role13,14. More recently, accelerated immune senescence, as evidenced by low ratios of CD4 to CD8 positive T cells (CD4/CD8 ratio), has been identified as a potential risk factor for NADM15,16. For example, in the US Veterans Aging Cohort Study, the CD4/CD8 ratio, but not the CD4 cell count, was an independent risk factor for lung cancer15.
We linked the database of the Swiss HIV Cohort Study (SHCS) with cantonal cancer registries to examine the relative importance of different risk factors for NADM, including immunological, virological and socio-behavioral factors.
The SHCS is an ongoing observational study that promotes clinical and public health research on HIV in Switzerland17,18. The SHCS is a collaboration between various hospitals, clinics, laboratories, and private practices across the country. Prospective enrollment started in 1988, and involves PLWHIV aged 16 years or above. Detailed socio-demographic and behavioral parameters for each patient are collected at enrollment. Laboratory results such as CD4+ and CD8+ cell count, HIV-1 RNA viral load or indicators of exposure to hepatitis B or C virus are recorded at enrollment and during follow-up visits. Detailed information on ART and other treatments, and of AIDS defining events are also collected at regular intervals. While NADM are recorded in the study, their ascertainment is incomplete.
Incident cancers in the SHCS were identified through linkage of SHCS records with the records of the Basel, Geneva, and Zurich cantonal cancer registries. At the time of the linkage, the data covered the period up to 2012 for Geneva and Zurich, and 2011 for Basel. Only SHCS participants registered in the areas covered by the three aforementioned cancer registries were included, representing about 65% of all participants. The linkage was done using privacy-preserving probabilistic record linkage19,20. Briefly, we employed a three-party protocol, with the SHCS and cancer registries collecting confidential individual-level patient data and the Institute of Social and Preventive Medicine at University of Bern serving as the independent linkage center. The linkage center supplied purpose-written encryption software21 that was used at SHCS centers and cancer registries to irreversibly encrypt personal identifying data (names, date of birth) using Bloom filters and calculate the similarity coefficients, which were then used to identify matching records at ISPM.
We supplemented the cancer cases reported in the SHCS by the cases identified through data linkage. We analyzed NADM classified according to the 10th revision of the International Classification of Diseases (ICD-10) that were diagnosed in about 40 or more patients: malignant neoplasm of anus and anal canal (C21), malignant neoplasm of liver and intrahepatic bile ducts (C22, excludes malignant neoplasm of other and unspecified parts of biliary tract), malignant neoplasm of trachea, bronchus and lung (C33–34), and prostate cancer (C61). We did not include Hodgkin lymphoma because most cases had been diagnosed in the early years of cART, when CD8 counts and viral load measurements were often missing.
We calculated standardized incidence ratios (SIRs) for each identified NADM by comparing the observed number of cancers among people with HIV with the expected number of occurrences, based on age-, sex- and period-specific rates for the Swiss population provided by the National Institute for Cancer Epidemiology and Registration (indirect standardization)22. We produced 95% confidence intervals (CI) for the SIRs assuming a Poisson distribution23.
We assessed the effect of baseline and time-updated exposure variables in Cox regression models. Time-updated variables included CD4 cell count (cells/µL), CD8 cell count (cells/µL), CD4/CD8 ratio and HIV viral load (copies/mL). We updated the immunological and virological factors at the start of each month. Months with no CD4 cell count, CD8 cell count, or HIV viral load were interpolated by carrying forward or carrying back the closest measured value within 12 months, provided there was no change in cART and no diagnosis of cancer in the interval. Time-updated bacterial pneumonia was additionally considered for lung cancer: history of bacterial pneumonia changed from no to yes when pneumonia was diagnosed. Other variables included age, likely HIV transmission group (men having sex with men [MSM], intravenous drug users [IDU], other men, other women), current smoking at enrollment (yes, no), highest completed level of education (compulsory, secondary, tertiary), chronic hepatitis B and exposure to hepatitis C (yes or no, as a time-updated variable), and calendar period (time-updated; before 2002, 2002–2007, 2008 and later). Patients were defined as having chronic hepatitis B if they had previously been tested positive for HBs-antigen or HBV-DNA, and as being exposed to hepatitis C during follow-up if they had tested positive for HCV-RNA.
We used Cox regression models for time to event analyses, measuring time from enrollment to the diagnosis of a NADM, the last clinic visit in the study period, or death, whichever came first. Follow-up time in patients enrolled before 1996, the year cART was introduced, was left truncated at 31 December 1995. Patients diagnosed with a NADM before 1 January 1996 were excluded from the analysis for that specific NADM.
In a first step, we modeled the association of the time-updated immunological and virological exposure variables (CD4 cell count, CD8 cell count, CD4/CD8 ratio, and HIV viral load) with each of the four cancers separately, adjusting for the other variables. To reduce the risk of detecting associations that reflect reverse causality, we lagged these variables for 12 months or more, and captured cumulative exposure using simple moving averages (SMA). We entered the following representations of each variable: lagged by 12, 24 or 36 months; SMA over 12 months - lagged by 12 or 24 months; SMA over 24 months - lagged by 12 months (see Figure 1). In a next step we selected variables and representations of variables for inclusion into a final, malignancy-specific model. We selected one representation of each immunological and virological variable based on the goodness of fit (indexed by the Akaike information criteria [AIC]) and carried over variables significantly associated with cancer incidence (P <0.05) when modeled separately. The intermediary and final models were adjusted for the variables age, transmission group, smoking, education, chronic hepatitis B, exposure to hepatitis C, and calendar period. The time-updated variables for bacterial pneumonia (used only in the lung cancer analysis), hepatitis B, and hepatitis C were lagged by 12 months.
We assessed proportionality assumptions of the Cox model using the Schoenfeld residuals. Results are shown as hazard ratios (HR) with 95% confidence intervals (95% CI). We imputed missing values for smoking using multivariate imputation by chained equations and pooled results of five imputed dataset. We present the analyses with imputation since results were similar to complete-case analyses. All analyses were done in R Project for Statistical Computing (version 3.6.0) software24 using packages survival (version 2.42), mice (version 3.30) and mitools (version 2.3). R code and explanatory documents are available as extended data25.
A total of 563 incident NADM were identified in the two data sources (SHCS and cancer registries). The SHCS recorded 60.9% of all cases, and the registries 76.2%. For the cancers analyzed in this study, the completeness of recording of cases in the SHCS was 54.3% for anal cancer, 66.7% for liver cancer, 71.4% for lung cancer, and 72.7% for prostate cancer. The corresponding percentages for the cancer registries were 85.7%, 80.6%, 85.7% and 93.2%, respectively (Table 1).
| Non AIDS Defining Malignancy (NADM) | Number of cases identified through linkage and in SHCS | Number of cases identified through linkage only (a) | Number of cases reported in SHCS only (b) | Total (c) | Completeness SHSCS (%)* | Completeness registries (%)& |
|---|---|---|---|---|---|---|
| Anus | 28 | 32 | 10 | 70 | 54.3 | 85.7 |
| Lung | 28 | 14 | 7 | 49 | 71.4 | 85.7 |
| Prostate | 29 | 12 | 3 | 44 | 72.7 | 93.2 |
| Liver | 17 | 12 | 7 | 36 | 66.7 | 80.6 |
| Head and neck | 7 | 23 | 2 | 32 | 28.1 | 93.8 |
| Hodgkin Lymphoma | 23 | 7 | 7 | 37 | 81.1 | 81.1 |
| Bladder | 3 | 9 | 0 | 12 | 25.0 | 100 |
| Breast | 9 | 3 | 4 | 16 | 81.3 | 75.0 |
| Colon and Rectum | 6 | 2 | 5 | 13 | 84.6 | 61.5 |
| Kidney | 5 | 0 | 4 | 9 | 100 | 55.6 |
| Oesophagus | 4 | 0 | 4 | 8 | 100 | 50.0 |
| Skin Melanoma | 6 | 13 | 5 | 24 | 45.8 | 79.2 |
| Stomach | 3 | 1 | 3 | 7 | 85.7 | 57.1 |
| Testis | 6 | 1 | 1 | 8 | 87.5 | 87.5 |
| Other NADM | 35 | 91 | 72 | 198 | 54.0 | 63.6 |
| All NADM | 209 | 220 | 124 | 563 | 60.9 | 76.2 |
Anal cancer was diagnosed in 70 patients over 71,592 person-years for an incidence rate of 9.78 per 10,000 person-years (95% confidence interval [CI] 7.62-12.35). Lung cancer was diagnosed in 49 patients over 71,888 person-years (6.82 per 10,000; 95% CI 5.04-9.01), prostate cancer in 44 men over 50,322 person-years (8.74 per 10,000; 95% CI 6.35-11.74), and liver cancer in 36 patients over 71,911 person-years (5.01 per 10,000; 95% CI 3.51-6.93).
Compared to the Swiss general population, anal, lung, and liver cancer all exhibited higher rates in the SHCS, whereas the rate of prostate cancer was similar (Figure 2). The difference was largest for anal cancer: the incidence was 76 times higher in people with HIV (SIR 76.1, 95% CI 60.2-96.2) than in the general Swiss population.
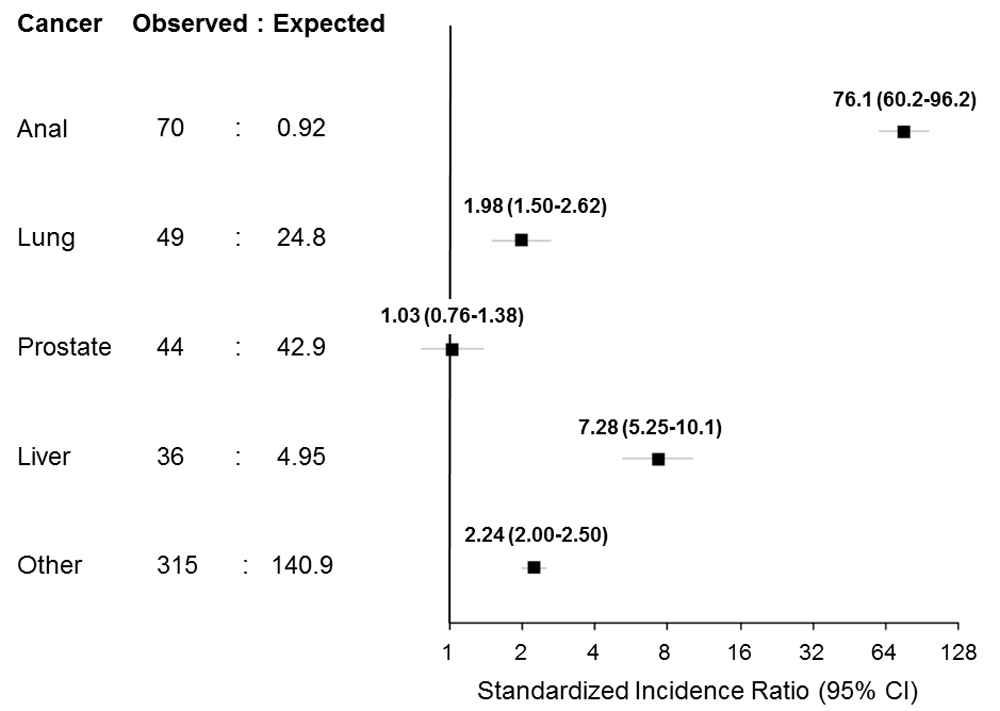
Characteristics of SHCS participants who developed cancer and participants who did not are shown in Table 2. Compared to those who remained free of cancer, HIV-positive individuals, who developed cancer were slightly older, more likely to be smokers, and at a more advanced clinical stage of HIV infection according to the Centers for Disease Control and Prevention classification26. The distribution across HIV transmission groups also varied between cancers: the majority of patients who developed anal or prostate cancer were MSM, while those with lung or liver cancer were predominantly patients with a history of IDU.
We selected one representation of each exposure variable based on goodness of fit models. The AIC values for the different models are shown in Table 3, the results of the separate models for each immunological and virological variable in Table 4 and the results of the mutually adjusted models in Table 5. For anal cancer, CD4 count, CD8 count and CD4/CD8 ratio were all associated with cancer risk in the separate models (Table 4). In the mutually adjusted model, a higher CD4 cell count (lagged 12 months) and a CD8 count below 1000 cells/µl (lagged by 24 months) continued to be significantly associated (P=0.002) with a reduced risk of anal cancer (Table 5). For example, in patients with a 12-month lagged CD4 count above 500 cells/µl, risk was reduced to about a fourth compared to individuals with a CD4 count below 200 cells/µl (hazard ratio [HR] 0.24; 95% CI 0.10-0.61). The risk of anal cancer was substantially higher in MSM (HR 6.86, 95% CI 1.64-28.6) than other men and higher in smokers (HR 2.07, 95% CI 1.07-3.98) compared to non-smokers.
Lag12, lagged by 12 months; SMA12: simple moving average over 12 months.
Models are adjusted for age, HIV transmission group, smoking, hepatitis B and C statuses, education, and calendar year, and include only one exposure variable. Numbers in bold highlight the representations selected for analysis.
| Anal cancer (n=70) | Lung cancer (n=49) | Prostate cancer (n=44) | Liver cancer (n=36) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| CD4 cell count (cells/µl) | <0.001 | 0.28 | 0.80 | 0.001 | ||||
| Lag | 12m | 24m | 24m | 12m, 24m SMA | ||||
| <200 | 1 | 1 | 1 | 1 | ||||
| 200-349 | 0.58 (0.29-1.14) | 1.06 (0.38-2.92)a | 0.73 (0.26-2.00) | 1.05 (0.44-2.49)a | ||||
| 350-499 | 0.32 (0.15-0.68) | 0.27 (0.08-0.95) | ||||||
| 500+ | 0.22 (0.11-0.46) | 1.54 (0.55-4.31) | 0.95 (0.38-2.36) | 0.06 (0.01-0.49) | ||||
| CD8 cell count (cells/µl) | 0.01 | 0.04 | 0.36 | 0.23 | ||||
| Lag | 24m | 36m | 12m, 12m SMA | 12m | ||||
| <1000 | 1 | 1 | 1 | 1 | ||||
| 1000+ | 1.90 (1.14-3.15) | 1.89 (1.03-3.47) | 1.34 (0.72-2.49) | 0.64 (0.29-1.42) | ||||
| CD4/CD8 ratio | <0.001 | 0.17 | 0.93 | 0.015 | ||||
| Lag | 12m, 24m SMA | 12m | 24m | 24m | ||||
| <0.50 | 1 | 1 | 1 | 1 | ||||
| 0.50-0.99 | 0.41 (0.22-0.75) | 0.99 (0.52-1.89) | 0.67 (0.32-1.39) | 0.51 (0.23-1.12) | ||||
| ≥1.00 | 0.08 (0.01-0.59) | 0.29 (0.07-1.26) | 1.14 (0.50-2.61) | 0.18 (0.02-1.37) | ||||
| HIV RNA (copies per ml) | 0.06 | 0.13 | 0.70 | 0.66 | ||||
| Lag | 12m, 12m SMA | 24m | 12m | 24m | ||||
| <50 | 1 | 1b | 1 | 1 | ||||
| 50-499 | 1.79 (0.77-4.21) | 0.24 (0.03-1.79) | 0.26 (0.03-1.92) | |||||
| ≥500 | 1.79 (0.96-3.33) | 1.67 (0.86-3.25) | 1.34 (0.62-2.90) | 0.91 (0.39-2.11) | ||||
| Bacterial pneumonia (lagged 12m) | na | 0.03 | na | na | ||||
| No | 1 | |||||||
| Yes | 3.23 (1.13-9.24) | |||||||
a estimate for CD4 cell count 200-499 cells/µl. b the reference category is <500 HIV RNA copies per ml.
HR, hazard ratio; CI, confidence interval; SMA, simple moving average; na, not applicable. Models for anal and liver cancer are adjusted for age, HIV transmission group, smoking, hepatitis B and C status, education, and calendar period. Models for lung cancer are stratified by age and adjusted for HIV transmission group, smoking, hepatitis C status, education, and calendar period. Models for prostate cancer are stratified for age and adjusted for HIV transmission group, smoking, hepatitis B and C status, education, and calendar period.
| Anal cancer (n=70) | Lung cancer (n=49) | Prostate cancer (n=44) | Liver cancer (n=36) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Exposure variables | ||||||||
| CD4 cell count (cells/µl) | 0.002 | 0.03 | ||||||
| Lag | 12m | 12m, 24m SMA | ||||||
| <200 | 1 | 1 | ||||||
| 200-349 | 0.58 (0.27-1.22) | 1.15 (0.47-2.81)a | ||||||
| 350-499 | 0.33 (0.14-0.76) | |||||||
| 500+ | 0.24 (0.10-0.61) | 0.08 (0.01-0.70) | ||||||
| CD8 cell count (cells/µl) | 0.04 | 0.04 | ||||||
| Lag | 24m | 36m | ||||||
| <1000 | 1 | 1 | ||||||
| 1000+ | 1.84 (1.02-3.30) | 1.89 (1.03-3.48) | ||||||
| CD4/CD8 ratio | 0.11 | 0.40 | ||||||
| Lag | 12m, 24m SMA | 24m | ||||||
| <0.50 | 1 | 1 | ||||||
| 0.50-0.99 | 0.74 (0.36-1.52) | 0.74 (0.33-1.66) | ||||||
| 1.00+ | 0.19 (0.02-1.51) | 0.57 (0.07-4.44) | ||||||
| Bacterial pneumonia (lagged 12m) | 0.50 | |||||||
| No | 1 | |||||||
| Yes | 1.67 (0.39-7.10) | |||||||
| Other variables | ||||||||
| HIV transmission group | <0.001 | 0.01 | 0.66 | 0.64 | ||||
| Other men | 1 | 0.72 (0.23-2.24) | 0.90 (0.46-1.76) | 0.67 (0.24-1.87)b | ||||
| Intravenous drug use | 1.05 (0.18-6.09) | 3.44 (1.42-8.35) | 0.41 (0.04-3.71) | 1.05 (0.38-2.95) | ||||
| MSM | 6.86 (1.64-28.59) | 1 | 1 | 1 | ||||
| Other women | 4.92 (1.05-23.06) | 2.34 (0.97-5.62) | NA | |||||
| Smoking at enrollment | 0.03 | 0.02 | 0.06 | 0.52 | ||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 2.07 (1.07-3.98) | 6.50 (1.36-31.2) | 1.92 (0.96-3.83) | 1.41 (0.51-3.95) | ||||
| Hepatitis B infection (positive for surface antigen or DNA, lagged 12m) | 0.53 | 0.72 | <0.001 | |||||
| No | 1 | 1 | 1 | |||||
| Yes | 1.36 (0.54-3.43) | 0.76 (0.18-3.19) | 7.86 (3.78-16.4) | |||||
| Hepatitis C infection (RNA positive, lagged 12m) | 0.23 | 0.14 | 0.58 | 0.002 | ||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.39 (0.09-1.77) | 0.45 (0.16-1.30) | 0.53 (0.06-4.61) | 4.77 (1.80-12.7) | ||||
a estimate for CD4 cell count 200-499, b estimate for other women or men
HR, hazard ratio; SMA, simple moving average; MSM, men having sex with men.
All models are adjusted for the variables shown and for age, calendar period, and highest completed education. Models for lung cancer and prostate cancer are stratified with respect to age.
For lung cancer and prostate cancer, Cox regression models were stratified by age to preserve proportional hazards. In the case of lung cancer, CD8 cell count (lagged by 36 months) was the only significant predictor (P=0.04) among the immunological and virological variables (Table 2): participants with CD8 cell counts equal to or above 1000 cells/µl had a greater risk of developing lung cancer, compared to those with counts below 1000 cells/µl (HR 1.89, 95% CI 1.03-3.48). A history of bacterial pneumonia was associated with lung cancer in the separate model (Table 4), but not after adjusting for CD8 cell count (Table 5). Smoking at enrollment was strongly associated with lung cancer (HR 6.50, 95% CI 1.36-31.2, Table 5). For prostate cancer, none of the immunological or virological were associated with a risk of cancer (Table 4), though there was some evidence of smoking being associated with higher rates (HR 1.92, 95% CI 0.96-3.83, Table 5).
For liver cancer, exposure to lower CD4 counts (SMA over 24 months, lagged by 12 months) and the CD4/CD8 ratio (lagged by 24 months) were associated with cancer incidence in the separate models (Table 4). In the final model (Table 5), only the CD4 cell count was predictive, with patients with average counts of 500 cells/µl or above at much lower risk than patients with counts below 200 cells/µL (HR 0.07, 95% CI 0.01-0.59). Chronic hepatitis B infection (HR 7.86, 95% CI 3.78-16.4) and hepatitis C infection (HR 4.77, 95% CI 1.80-12.7) were both strongly associated with liver cancer (Table 5).
In the era of cART, life expectancy of PLWHIV is approaching that of the general population, the incidence of AIDS-defining malignancies has declined sharply, and NADM have emerged as an important co-morbidity1,2,27,28. This study linked the database of the SHCS with three cantonal cancer registries to identify incident NADM. Anal, lung, prostate and liver cancers were common NADM among participants of SHCS. Compared to the general population, three of the four cancers occurred more frequently among PLWHIV. The exception was prostate cancer, whose incidence was similar to the general population. Associations of lagged and cumulative exposure to immunodeficiency or immune senescence were evident for some but not all cancers, and HIV replication was not related to cancer risk.
The SHCS is a well-established cohort of people living with HIV in Switzerland, where regular follow-up visits provide a wealth of information to inform fundamental, clinical and epidemiological research17,18. Both genders and the various transmission groups are well represented. We used state-of-the-art privacy preserving linkage to complement incident cancers recorded in the SHCS with cases from cantonal registries19,20. Interestingly, neither the SHCS nor the cancer registries had a complete record of cases. This situation should improve in the near future, with the introduction of nationwide, compulsory cancer registration in Switzerland in 202029. Previous analyses of cancer risk in the SHCS used a matched case-control approach, which precluded the study of some risk factors30,31. For example, matching cancer cases and controls on transmission group hampered the evaluation of hepatitis virus infections for liver cancer31, or of smoking for anal cancer32. Our study also has limitations. Cancer linkage was only possible for three cantonal registries, which meant that about 35% of SHCS participants could not be included, limiting statistical power. Anal intercourse is a risk factor for anal cancer, and excessive alcohol consumption for liver cancer, but data on sexual behavior and alcohol have been collected in the SHCS only since 2000 and 2005, respectively, and were not considered here.
The reasons for the higher cancer incidence in PLWHIV differ across cancers, and include multiple factors. Several studies have shown that immunodeficiency was associated with a higher risk of NADM28,33–35. Some of these studies analyzed NADM as a single group, or as groups of infection-related and non-infection-related NADM. We found that immunodeficiency (indexed by exposure to low CD4 counts) was associated with anal cancer and liver cancer, but not with lung cancer or prostate cancer. Our results indicate that each NADM should be analyzed separately, rather than combining different NADM into groups.
For lung cancer, a high CD8 cell count several years prior to the diagnosis was a risk factor whereas a low CD4 count was not associated with lung cancer. Persistent CD8 cell elevation among HIV patients on long-term cART, a marker of immune senescence, is associated with inflammatory, non-AIDS related events such as cardiovascular, renal, respiratory, metabolic diseases and NADM, and with increased non-AIDS-related mortality36–38. Mussini and colleagues suggested that the CD4/CD8 ratio might be a more robust marker of the immune dysfunction associated with NADM than the CD4 or the CD8 cell count39. Indeed, a study of US veterans living with HIV found that the CD4/CD8 ratio was the strongest predictor of incidence with CD4/CD8 ratio ratios below 1.0 associated with higher risk15. In contrast to the veterans study, the CD8 cell count was more robustly associated with lung cancer risk than either the CD4 cell count or the CD4/CD8 ratio in our analysis, suggesting that in the cART era, immune senescence rather than immunodeficiency increases the risk of lung cancer.
A history of recurrent pneumonia was associated with lung cancer risk in the HIV/AIDS Cancer Match study40 and in the US Veterans Aging Cohort Study15. In our analyses, the time-updated history of bacterial pneumonia was associated with lung cancer risk in the model adjusted for baseline variables, but evidence of this association disappeared after adjusting for CD8 count. Clearly, the most important risk factor for lung cancer is smoking, which in Switzerland and elsewhere is substantially more common among PLWHIV than in the general population41–43. Smoking was also associated with anal cancer, but it remains unclear whether the association is causal or whether smoking is a marker for unsafe sexual behavior and exposure to HPV. Several studies have found that smoking may be associated with a higher risk of HPV infection44–46.
Prostate cancer is one of the most common malignancies among men in Western populations, and an important cause of cancer death. Older age, a positive family history, black race and elevated levels of insulin growth factor (IGF)-I have been shown to be risk factors for prostate cancer47,48. Our results do not suggest that immunodeficiency or immune senescence strongly influence prostate cancer risk. In this context, it is noteworthy that prostate cancer is not consistently responsive to immune therapy, in contrast to other solid tumors, including lung cancer49.
Liver cancer was associated with prolonged exposure to CD4 counts below 500 cells/µl however; chronic viral hepatitis was a likely cause in almost all patients developing liver cancer: there was evidence of chronic HBV or HCV infection in 83% of patients with liver cancer. HBV and HCV were both strong risk factors for the cancer. An explanation for the strong association with HBV might be hepatitis delta virus (HDV) co-infection, which in the SHCS is observed in about 15% of patients with chronic HBV infection50. HDV infection accelerates the progression of HBV related liver disease, including hepatocellular cancer50.
Immunodeficiency and, to a lesser extent, immune senescence are prevented by starting cART as soon as possible after HIV diagnosis36, as recommended by the World Health Organization51. The “Universal Test and Treat” approach to HIV coupled with interventions to ensure adherence will likely reduce the incidence of some NADM. The integration of smoking cessation interventions into routine care of people living with HIV is another important measure52. All SHCS participants are tested for chronic HBV infection to guide decisions on HBV vaccination and on the inclusion antiretrovirals with activity against HBV in cART regimens, as recommended by guidelines53,54. Similarly, testing for antibodies against HCV is done at HIV diagnosis and annually thereafter, and there is universal access to direct acting anti-virus (DAA) therapy against HCV. Future analyses of the incidence of NADM in the SHCS will document the impact of these measures. Of note, the effectiveness of anal cancer screening is unclear: the ongoing Anal Cancer/HSIL Outcomes Research Study (ANCHOR) aims to determine whether treatment of high grade squamous intraepithelial lesions (HSIL) prevents anal cancer55.
The importance of immunodeficiency and immune senescence, and of other risk factors differs across NADM, with important implications for prevention. Immunodeficiency was an important risk factor for anal and liver cancer whereas immune senescence was associated with lung cancer and anal cancer.
The SHCS was approved by the Ethics committees of the participating institutions. At enrolment, SHCS participants provide written informed consent for the use of biological and clinical data. For the present study, we obtained additional ethical approval for privacy-preserving probabilistic record linkage from the Ethics Committee of the Canton of Bern.
The National Institute for Cancer Epidemiology and Registration (NICER) is happy to share the anonymized cancer registry data with eligible partners. If you wish to receive specific data from the NICER database, please complete the respective form at https://www.nicer.org/en/data/request-data/.
The data analyzed in this study are sensitive, having been contributed by people living with HIV. Access to de-identified data is restricted to collaborative projects that have been submitted to and approved by the Scientific Board of the SHCS. Please contact the SHCS at http://www.shcs.ch/contact if you are interested in gaining access to the study data. Sharing or linkage of data may require additional ethics approval.
Files with the R code used in the analysis are provided as extended data, along with an explanatory document, on Open Science Framework
Open Science Framework: Extended data for Non-AIDS defining malignancies in the combination ART era: immunological and socio-behavioral risk factors (Ruffieux et al. Faculty1000research 2019). https://doi.org/10.17605/OSF.IO/GY5VM25
This project contains the following extended data:
code.description final.pdf (description of R code)
pilot.R (initializes data preprocessing for a given cancer)
preprocess_SHCS.R (loads SHCS data)
preprocess_linkage.R (loads the cases of cancer that could be linked to the SHCS)
merge.R (appends patient-level information, creates dataset with one line per patient)
risk_factors_monthly.R (creates dataset with one line per month, per patient, with time-updated variables)
write_res_df.R (sets up data for survival analysis for a specific cancer)
cancer_counts.R (produces the number of cancers per data source reported in Table 1)
patient_characteristics.R (produces results in Table 2)
crude_incidence_rates.R (produces crude cancer incidence rates)
SIR_NADCs.R (produces the SMRs shown in Figure 2)
impute_smoking.R (multiple imputation of smoking variable)
Cox_model_selection.R (produces the AICs in Table 3).
risk_factors_Cox.R (Cox regression to produce results in Table 4 and Table 5)
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The linkage and analysis of the data were funded by grants from Cancer Research Switzerland [KFS-3165-02-2013 and KFS-3862-02-2016]. The SHCS is supported by the Swiss National Science Foundation (SNSF) [177499]. ME is supported by special project funding from the SNSF [174281].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful to the patients, study nurses, and care providers who participate in the SHCS.
The members of the Swiss HIV Cohort Study are: Aebi-Popp K, Anagnostopoulos A, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the SHCS), Haerry D (deputy of "Positive Council"), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Rudin C, Scherrer AU (Head of Data Centre), Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Trkola A, Vernazza P, Wandeler G, Weber R, Yerly S.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: I was the lead author in the similar study from the Veterans Aging Cohort Study that was cited in this study; this paper uses very similar methods to our paper.
Reviewer Expertise: HIV and cancer risk/outcomes
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Aug 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)