Keywords
air trapping, COPD, exercise, isocapnic buffering phase, ventilatory equivalent
air trapping, COPD, exercise, isocapnic buffering phase, ventilatory equivalent
BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second; GOLD, global initiative for chronic obstructive lung disease; IC, inspiratory capacity; RV, residual volume; TLC, total lung capacity; VCO2, CO2 output; VE, minute ventilation; VE/VCO2, ventilatory equivalent for CO2; VE/VO2, ventilatory equivalent for oxygen; ΔVE/VCO2, nadir to peak value of VE/VCO2; ΔVE/VO2, nadir to peak value of VE/VO2; VO2, oxygen consumption; VT, ventilatory threshold.
Chronic obstructive pulmonary disease (COPD) patients often demonstrate significant effort limitation, chiefly resulting from gas exchange abnormalities and ventilation-perfusion mismatching1. This situation is often compounded by hyperinflation and air trapping with dynamic hyperinflation during exercise and gradual reduction of inspiratory capacity2. To evaluate exercise capacity and determine the degree of effort limitation and its mechanisms, the incremental exercise test is often applied, during which several ventilatory events occur and draw close attention.
During incremental cardiopulmonary exercise testing, the ratio of minute ventilation (VE) to carbon dioxide output (VCO2) and to oxygen consumption (VO2), also known as ventilatory equivalent for carbon dioxide (VE/VCO2) and oxygen (VE/VO2), respectively, serve to evaluate ventilatory efficiency. The ventilatory threshold (VT), a term coined in the context of gas exchange measurements and represents to a great extent the VO2 at anaerobic threshold, heralds the onset of the isocapnic buffering phase, in which there is an increased contribution of anaerobic metabolism to provide the energy required for the increasing demands of exercise, accompanied by increased CO2 production with corresponding ventilation increase. With the accumulation of lactic acid and H+ protons, as the bicarbonate reserves are exhausted beyond the isocapnic buffering phase, an augmented ventilatory response starts at the ventilatory compensation point, which is disproportionate to the degree of CO2 production, leading to VE/VCO2 surge towards peak effort, a phenomenon termed the ventilatory compensation phase.
The VE/VCO2 is often increased as a result of ventilation-perfusion mismatching1,3. The nadir VE/VCO2 value during incremental exercise is inversely related to the degree of effort limitation4, as well as the severity of airway obstruction, as determined by the values of forced expiratory volume in first second (FEV1)5. The nadir value is considered to reflect ventilatory efficiency as higher values obtained at the early stages of the incremental exercise test result from frequently encountered hyperventilation. Interestingly, marked hyperinflation and reduced ventilatory capacity tend to reduce ventilatory equivalents as ventilation may become constrained. Both baseline and peak effort VE/VCO2 values were found to be lower in patients with more severe airway obstruction as VE is decreased relative to VCO2 in these patients6.
Although COPD patients may terminate exercising at an early stage during the incremental exercise test due to airflow obstruction and air trapping, suboptimal effort and deconditioning, the latter of which may be associated with somewhat earlier development of metabolic acidosis, may also lead to early termination of the exercise test. Regardless of the reason causing decreased exercise performance, the ventilatory compensation phase may not be demonstrated. Ventilatory equivalents during incremental exercise may be affected by the above-mentioned processes in different directions. In this study we sought to confirm the presumption that the ability to achieve ventilatory compensation in response to acidosis is related to the degree of air trapping, with the hypothesis that the ability to augment ventilation beyond the ventilatory compensation point in COPD patients is inversely correlated with the degree of air trapping.
A retrospective analysis of data obtained from the medical records of COPD patients who underwent incremental cardiopulmonary exercise testing in the pulmonary function laboratory of the Institute of Pulmonary Medicine in the Hadassah Medical Center, Jerusalem, Israel between June 2010 and August 2016, and in whom whole body plethysmography was performed concurrently, as part of their routine clinical evaluation. The number of patients was determined by the availability of incremental exercise test and plethysmography data that was performed concurrently. Subjects were previously diagnosed with COPD based on post-bronchodilator spirometry showing an FEV1 to forced vital capacity (FVC) ratio (FEV1/FVC) of ≤0.7, regardless of the value of FEV11 (according to 2017 GOLD guidelines). Comorbidities such as obesity, cardiac disease and use of beta blockers were noted, but were not a cause to exclude patients from the study. The research ethics board (REB) of the Hadassah Medical Organization (protocol No. 0040-16-HMO) approved data collection and waived the need for informed consent, as this research was retrospective, did not affect patient management, or involve collecting biospecimens.
Pulmonary function tests and exercise tests were performed by technicians in the presence of a physician during exercise tests. Whole-body plethysmography was performed using a commercially available body plethysmograph (Elite series, MedGraphics). Spirometry performance and slow vital capacity determination were followed by assessment of lung volumes, which was performed by direct measurement of thoracic gas volume (TGV), from which the residual volume (RV), total lung capacity (TLC) and RV/TLC ratios could be calculated as the primary method to estimate gas trapping. Additionally, we determined the ratio of inspiratory capacity (IC) to total lung capacity, IC/TLC, which has been proposed to reflect lung expansion as a result of reduced lung recoil in emphysema7. Using the same lung function system, transfer factor of the lung for carbon monoxide (TLCO) was measured by the single breath method.
Patients performed incremental symptom-limited cycle ergometry connected to a metabolic system with cycle ergometer (Ultima CardiO2, MedGraphics). For each patient, baseline measurements were obtained during the resting period of 1 minute, after which the patient would start cycling at a constant rate of 50–60 rpm. Following a 1–2 minute warm-up period at 0 watts and with VO2 and VCO2 reaching a plateau, a ramp protocol was started with a workload increase of 15–25 watt per minute, depending on the predicted maximal VO2 and the general state of the patient. The test was terminated at exhaustion. Routine precordial 12-lead electrocardiogram monitoring, continuous measurements of VE, VO2, VCO2 (averaged every 10 seconds), heart rate and finger arterial pulse oximetry were recorded. The peak VO2 and the VT were both noted, as well as nadir ventilatory equivalents (VE/VCO2 and VE/VO2). Values of VE/VCO2 and VE/VO2 obtained at the termination of loaded exercise (peak effort) enabled calculation of the difference between peak effort value and nadir values of VE/VCO2 and VE/VO2 (designated ΔVE/VCO2 and ΔVE/VO2, respectively).
Statistical analysis was performed using the GraphPad Prism 3.0 program software and Spearman’s test was used for correlation analysis, with calculation of the Pearson correlation coefficient (r) between measured parameters. Correlations were considered of statistical importance if two-tailed p value was <0.05. Unpaired t-test was used to compare between groups, and a two-tailed p value < 0.05 was considered statistically significant
In total, 20 patients were included in our analysis with a mean ± SD age of 63 ± 10 years (range 37–77). None of the patients had cardiac failure. Two patients were receiving beta blockers. Table 1 summarizes clinical data of these patients.
| Age (years) | 63 ± 10 |
| Gender (% of males) | 80 |
| BMI (kg/m2) | 27.2 ± 6.8 |
| Static pulmonary function tests | |
| FEV1 (L) | 1.80 ± 0.70 |
| FEV1 (% predicted) | 63 ± 21 |
| FVC (L) | 3.07 ± 1.03 |
| FVC (% predicted) | 85 ± 22 |
| FEV1/FVC (%) | 59 ± 12 |
| RV (L) | 3.82 ± 1.82 |
| RV (% predicted) | 166 ± 60 |
| IC (L) | 2.1 ± 0.6 |
| IC (% predicted) | 73 ± 21 |
| TLC (L) | 6.94 ± 1.81 |
| TLC (% predicted) | 111 ± 24 |
| RV/TLC (%) | 55 ± 11 |
| IC/TLC (%) | 31 ± 8 |
| TLCO (% predicted) | 67 ± 17 |
|
No. of patients according to GOLD classification (% of all patients) | |
| GOLD I | 7 (35%) |
| GOLD II | 8 (40%) |
| GOLD III | 4 (20%) |
| GOLD IV | 1 (5%) |
| Exercise test | |
| Peak VO2 (% predicted maximal VO2) | 68 ± 15 |
| Nadir VE/VCO2 | 36 ± 7 |
| Peak effort VE/VCO2 | 39 ± 8 |
| ΔVE/VCO2 | 2.6 ± 3.4 |
| Nadir VE/VO2 | 37 ± 7 |
| Peak effort VE/VO2 | 45 ± 10 |
| ΔVE/VO2 | 8.0 ± 7.6 |
| Peak heart rate (% predicted maximal heart rate) | 80 ± 11 |
| Breathing reserve* (%) | 30 ± 15 |
| Arterial blood O2 desaturation at peak effort (%) | 2.4 ± 3.6 |
Values are mean ± SD.
BMI, body mass index; FEV1, forced expiratory volume; FVC, forced vital capacity; HR, heart rate; GOLD, Global initiative for chronic Obstructive Lung Disease; IC, inspiratory capacity; RV, residual volume; TLC, total lung capacity; TLCO, transfer factor of the lung for carbon monoxide; VO2, oxygen consumption; VE/VO2, ventilatory equivalent for oxygen; VE/VCO2, ventilatory equivalent for carbon dioxide; Δ is the difference between measured value of ventilatory equivalent at peak effort and nadir value.
*Breathing reserve = (maximal voluntary ventilation - peak ventilation) / maximal voluntary ventilation.
We found a statistically significant inverse correlation between both ΔVE/VCO2 (r = -0.5058, 95% CI -0.7750 to -0.08149, p = 0.0234) and ΔVE/VO2 (r = -0.5588, 95% CI -0.8029 to -0.1545, p = 0.0104), with the degree of air trapping as estimated by the RV/TLC ratio (Figure 1). On the contrary, there was no correlation between the nadir values of the ventilatory equivalents and RV/TLC (Table 2). Interestingly, inspiratory capacity to total lung capacity ratio (IC/TLC), which reflects pulmonary expansion in emphysema, did not show a correlation with either ΔVE/VCO2 or ΔVE/VO2 (Table 2).
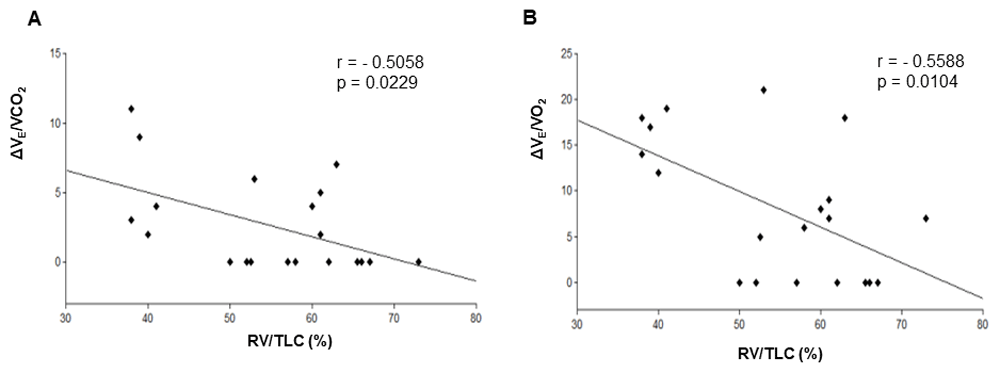
Correlation between ΔVE/VCO2 (A), ΔVE/VO2 (B) and RV/TLC. RV/TLC, residual volume to total lung capacity ratio; ΔVE/VCO2, nadir to peak increment of ventilatory equivalent for CO2; ΔVE/VO2, nadir to peak increment of ventilatory equivalent for oxygen. p<0.05 points to a statistically significant correlation.
p value <0.05 is considered of statistical importance.
r, Pearson correlation coefficient; BMI, body mass index; IC, inspiratory capacity; RV, residual volume; TLC, total lung capacity; FEV1, forced expiratory volume; TLCO, transfer factor of the lung for carbon monoxide; VO2, oxygen consumption; VE/VCO2, ventilatory equivalent for carbon dioxide; VE/VO2, ventilatory equivalent for oxygen; Δ is the difference between measured value at peak effort and nadir value
We examined possible correlation between ΔVE/VCO2 and other static parameters of pulmonary function or findings on exercise testing (summarized in Table 2). Notably, we found no significant correlation between neither ΔVE/VCO2 nor nadir VE/VCO2 and peak VO2 or FEV1. As expected1, nadir values of VE/VCO2 correlated with TLCO (r = -0.5281, 95% CI -0.7923 to -0.09708, p = 0.0201); however, we did not find a correlation between ΔVE/VCO2 and TLCO (Table 2).
In an attempt to identify reasons for the lack of increment of ΔVE/VCO2 besides factors related to air trapping, we compared values of parameters related to static pulmonary functions and those related to performance of exercise in patients with and without increment in VE/VCO2 (i.e., ΔVE/VCO2 = 0) (Table 3). While FEV1, IC/TLC, TLCO, peak VO2, and nadir VE/VCO2 were not different between these two groups, RV/TLC and peak respiratory exchange ratio had a statistically important difference between the two groups. However, these latter two factors are inherently derived from the parameters that the correlation study had examined and therefore, this difference is not surprising.
Values are mean ± SD. p<0.05 signifies an important difference.
RER, respiratory exchange ratio; RV, residual volume; TLC, total lung capacity; TLCO, transfer factor of the lung for carbon monoxide; VO2, oxygen consumption; FEV1, forced expiratory volume; IC, inspiratory capacity; VE/VCO2, ventilatory equivalent for CO2; ΔVE/VCO2 is the difference between measured value at peak effort and nadir value of VE/VCO2.
Inefficient ventilation in patients with COPD and particularly emphysema is often evidenced by elevated nadir VE/VCO2. The elevation of nadir VE/VCO2 may be related to the extent and severity of emphysematous changes in the lungs. It has been shown that in patients with mild to moderate airflow decrease, the values of nadir VE/VCO2 correlated with the percentage of low attenuation areas on computerized tomography8,9 as well as the decrease in pulmonary perfusion, as estimated by the inert gas rebreathing method9. Interestingly, in smokers without COPD, the degree of CO diffusion decrease correlated with elevation of nadir VE/VCO2 during the incremental exercise test10, again pointing to a strong relation between impaired ventilation efficiency and gas exchange ability. Notably, significantly increased ventilatory constraints, as occurs in marked air trapping, decrease VE, thus leading to lower nadir VE/VCO2 in more severe airway obstruction2,6.
In this study we examined the ability of COPD patients during incremental exercise testing to increase ventilation towards peak effort, focusing on the increase in the VE/VCO2 and VE/VO2 slopes that occur following the ventilatory compensation point. We quantified this phenomenon measuring the difference between the slope values of VE/VCO2 and VE/VO2 at nadir and peak effort obtained post the ventilatory compensation point, which we termed ΔVE/VCO2 and ΔVE/VO2, respectively. We found that the ability to increase ventilation during incremental exercise testing in response to metabolic acidosis, as normally occurs in higher exercise intensities beyond the ventilatory compensation point, was diminished in COPD patients and in correlation with the severity of air trapping as represented by RV/TLC obtained at rest. Therefore, inability to compensate for the increased ventilatory demands of acidosis may contribute to termination of effort at lower exercise intensity. Furthermore, this may implicate a difficulty in coping with the ventilatory demands of metabolic acidosis resulting from states such as acute renal failure or septic shock, thus ΔVE/VCO2 (and seemingly ΔVE/VO2) may allow assessment of the ability of COPD patients to withstand such metabolic challenges.
One way in which pulmonary hyperinflation can be expressed is by the increased ratio of RV/TLC, which reflects air trapping and is associated with airway narrowing. Alternatively, IC/TLC may also be decreased because of lung expansion associated with emphysematous changes and reduced lung recoil. A recent study showed that either increased RV/TLC or decreased IC/TLC or both simultaneously can be present, with airway narrowing on imaging being associated with increased RV/TLC and emphysema associated with deceased IC/TLC7. Therefore, it seems that the lack of increments in ventilatory equivalents in our study can be related to air trapping but not to the mere presence of emphysema.
One may argue that the limited rise of ventilatory equivalents beyond the ventilatory threshold during the incremental exercise test may reflect sub-optimal exercise performance; however, we think that the lower respiratory exchange ratio at peak exercise represents another manifestation of the decreased ventilatory response.
Although both ΔVE/VO2 and ΔVE/VCO2 seem to show similar correlation to RV/TLC and utility in estimating the ventilation response to acidosis under these conditions, a further study with larger numbers of patients may help distinguish the different roles of these parameters.
This study has several limitations. The retrospective design and the small number of patients included are important limitations, in particular when comparing two subgroups. However, these limitations may be of lesser significance when searching for correlations. Some patients were obese, which may have caused mild restriction, somewhat contributing to air trapping. However, we didn’t find a correlation between body mass index (BMI) and either ΔVE/VCO2 or ΔVE/VO2. The group with the greater ventilatory equivalents increment had a nearly significant higher nadir VE/VCO2 value (p = 0.53), suggesting a possible baseline difference between those who develop an increment versus those who don’t. This could be explained along the same line in which it is suggested that those patients who can develop an augmented ventilatory response, i.e. increase VE, are also able to produce a ventilatory equivalent increment towards peak effort. Unfortunately, dynamic hyperinflation, a well-known phenomenon that is associated with ventilatory constraints during exercise in COPD, was not assessed and therefore, we cannot comment on the relation between dynamic hyperinflation (i.e. changes in IC/TLC) and ventilatory equivalents increment in this cohort.
In conclusion, ventilation augmentation during incremental exercise testing at exercise intensities beyond the ventilatory compensation point in COPD patients was diminished in correlation with the severity of air trapping. Future studies will confirm the clinical usefulness of measuring ΔVE/VCO2 and ΔVE/VO2, including their potential role as serially measured physiologic parameters to assess effects of interventions (e.g. pulmonary rehabilitation) in COPD.
Harvard Dataverse: Ventilatory Equivalents increment during Exercie in COPD. https://doi.org/10.7910/DVN/QTCV0M11.
This project contains the following underlying data:
- nadir to peak VE-VCO2 during exercise in COPD.tab (demographic information and parameter measurements for each patient).
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Exercise tests and static pulmonary function tests were performed by medical technicians Ahuva Mizrachi, Ruhama Erental, Elat Bardach and supervised by Samir Nusair.
Note: This work has been presented in part, in poster form, during the European Respiratory Society International Congress 2016, London, United Kingdom.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Casaburi R, Patessio A, Ioli F, Zanaboni S, et al.: Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease.Am Rev Respir Dis. 1991; 143 (1): 9-18 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Exercise Physiology, COPD, Pulmonary Function Testing
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
References
1. Paoletti P, De Filippis F, Fraioli F, Cinquanta A, et al.: Cardiopulmonary exercise testing (CPET) in pulmonary emphysema.Respir Physiol Neurobiol. 2011; 179 (2-3): 167-73 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Exercise Physiology - Hypoxia - Ventilatory Response to Exercise and High Altitude - Critical Care and Emergency Medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 10 Mar 20 |
read | read |
|
Version 1 19 Sep 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)