Keywords
autism, pten, macrocephaly, ASD, sensorimotor
autism, pten, macrocephaly, ASD, sensorimotor
Sensorimotor gating is the ability of a sensory stimulus to suppress a motor response1. It can be measured by assessing prepulse inhibition (PPI), wherein a weak auditory stimulus inhibits a startle response that is induced by the following presentation of a loud sound2. Deficits in PPI have been widely reported in various neurological conditions, including autism spectrum disorder (ASD)3–5. Similar to humans, impairments in PPI have been reported in ASD models such as Fmr1 and Cntnap2-knockout (KO) mice; however, the underlying mechanism is unknown6,7. Pten mutant mice are another model of autism and can be used to investigate the connection between a cell signaling pathway commonly implicated in ASD, the PI3K/AKT/mTOR pathway, and specific autistic-like deficits8. In the present study, we use neuronal subset-specific (NS)-Pten KO mice that exhibit hyperactivation of the PI3K/AKT/mTOR pathway in the cortex, hippocampus, and cerebellum, and assess PPI in order to further elucidate the potential relationship between PI3K/AKT/mTOR signaling and deficits in sensorimotor gating9.
Male and female mice on a FVB based mixed background were obtained from Baylor College of Medicine and have been bred for more than 10 generations at Baylor University. Heterozygous NS-Pten males (n=6) and females (n=12) were used to breed NS-Pten wildtype (WT) and KO pups (RRID: MGI:3714016). The housing for the breeders consisted of two females housed with one male. Genotype was determined from toe clippings taken on postnatal day (PD) 10 (performed by Mouse Genotype, Escondido, CA, USA). On PD 21, animals were weaned and housed with mixed genotype littermates in groups of n=3–5 in cages (Allentown Caging PC7115HT, Allentown, PA, USA) filled with sani-chip bedding (7090 Teklad, Envigo, Somerset, NJ, USA) kept in a room on a 12-hr light/dark diurnal cycle held at 22°C. Mice had ad libitum access to food and water. All animals were tested at 9–10 weeks of age between the hours of 10:00 and 11:30 a.m. A total of 29 male mice were assessed, 17 NS-Pten KO and 12 WT mice. The target sample size was determined by, and is in accordance with, the PPI literature10–12. The final sample sizes were as follows: day 1: n=12 WT, n=17 KO, day 2: n=12 WT, n=13 KO, day 3: n=9 WT, n=9 KO. All test procedures were carried out in compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by Baylor University’s Institutional Animal Care and Use Committee. Once the experiment concluded, mice were placed into a CO2 chamber and euthanized.
Sensorimotor gating was assessed via the SR-LAB system, which consists of a 15 × 14 × 18 inch isolation cabinet, a plexiglass cylinder (3.2-cm diameter) mounted on a sensor platform, a standard speaker used to generate white noise, and a high-frequency speaker used to generate stimuli (San Diego Instruments, San Diego, CA, USA). The paradigm consisted of three separate testing days: habituation, prepulse inhibition, and startle response, and was conducted as previously described6.Each test day is further detailed in the Figure 1 legend. To eliminate potential confounds during testing, background sound levels were maintained at 68 dB and the experimenter was not present.
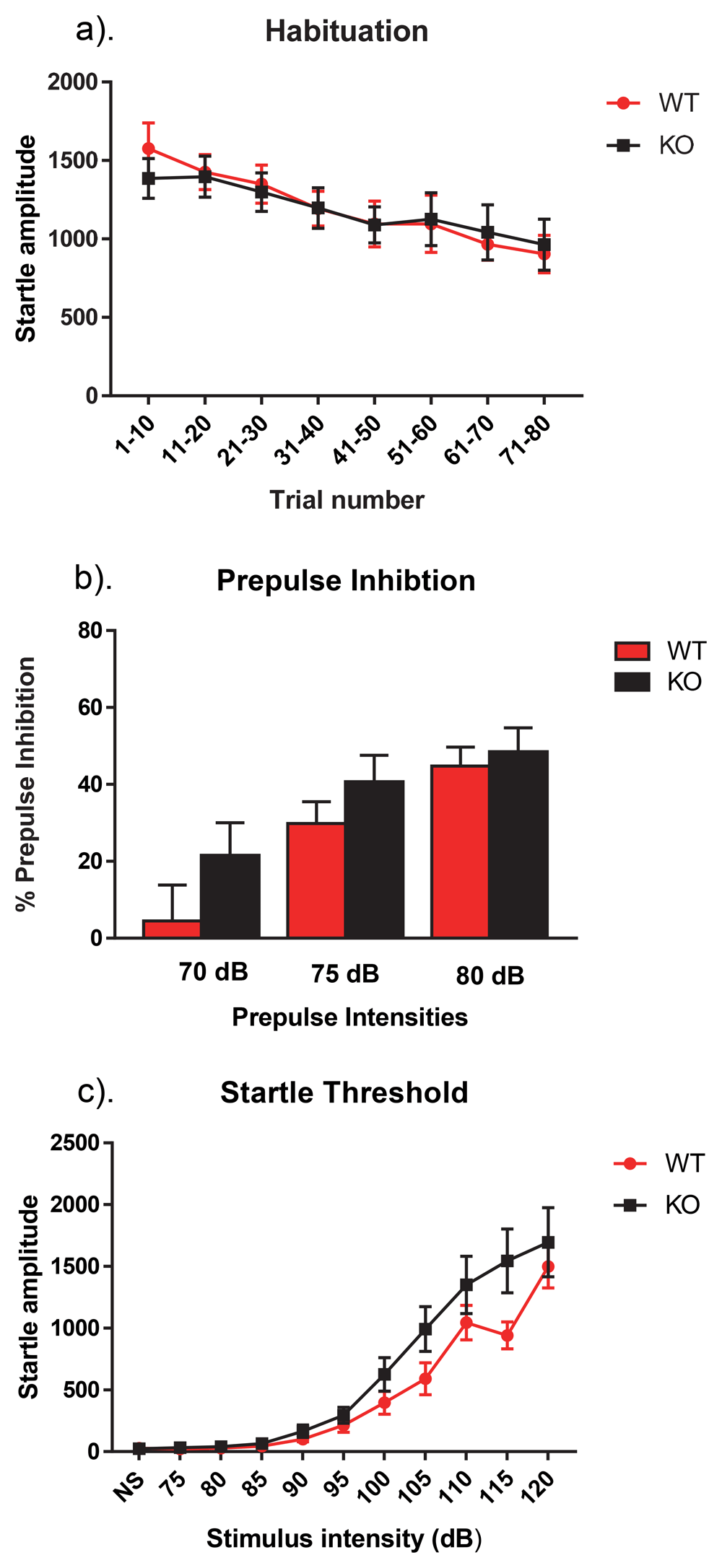
(a) On the first day of testing, the animal was acclimated to the room for 30 minutes then was placed inside the cylinder for a 5-minute habituation period, which was followed by 80 startle stimuli delivered at a fixed interval of 15 seconds. The startle stimulus was a 40-ms, 120-dB noise burst, with a rise/fall time of less than 1 ms. We found that there was no significant difference in habituation between KO and WT mice (p > 0.05). (b) Day 2 of testing occurred 24 hours after day 1 and tested prepulse inhibition. Once the mice were in the apparatus, there was a 5-minute habituation phase that was followed by 20 presentations of a 40-ms, 120-dB noise burst that had a fall time of less than 1 ms. In the prepulse phase, mice were presented with 90 trials consisting of three prepulse intensities, 70, 75, and 80 dB. Each prepulse was 20 ms in duration with a rise/fall time of less than 1 ms and were spaced 15 seconds apart. We found no difference in the percentage of prepulse inhibition between groups following prepulses of 70, 75, or 80 dB (p > 0.05). (c) One week after the prepulse session, the startle threshold was assessed. Following the 5-minute habituation period, the mice were presented with 99 trials of 11 trial types. These include a no stimulus trial and 10 startle stimuli trials ranging from 75–120 dB at 5 dB intervals. The startle stimuli are 40 ms noise bursts with a rise/fall of less than 1 ms. The 11 trial types were pseudorandomized, with each trial type being presented once in a block of the 11 trials. We observed no difference in startle threshold between NS-Pten KO and WT mice (p > 0.05). Data are presented as the mean ± standard error of the mean (SEM).
GraphPad Prism 7 software (La Jolla, CA) or SPSS 21.0 (IBM, USA) were used to analyze the data. Repeated-measure ANOVAs were run for habituation, prepulse inhibition, and startle threshold. No post-hoc tests were performed. A total of n=4 KO mice were excluded from the day 2 analysis and n=11 mice (3 WT and 8 KO) were excluded from the day 3 analysis due to protocol malfunction or death as a result of the severity of the knockout. A value of p < 0.05 was considered significant for each statistical test.
When assessing the sensorimotor gating paradigm, no main effects were found for habituation (F[1,27] = 0.17, p >0.05), prepulse inhibition (F[1,23] = 2.65, p >0.05) or startle threshold (F[1,16] = 2.33, p >0.05). There were also no interactions for habituation (F[7,189] = 0.91, p >0.05), prepulse inhibition (F[2,46] = 0.71, p >0.05), or startle threshold (F[10,160] = 1.94, p >0.05) (Figure 1a–c). Raw results for each procedure on each day for every animal are available as Underlying data13.
The NS-Pten KO mice did not exhibit significantly different sensorimotor gating from WT mice. A previous study by Kwon et al. (2006) assessed neuron-specific enolase (Nse)-Pten KO mice in a variation of the PPI protocol and reported a decrease in percent inhibition at 4 dB but no differences at 8 or 16 dB10. Our study assessed percent inhibition at 70, 75, and 80 dB, per established protocol, and found no differences at these intensity6. Therefore, this indicates that there may only be changes in percent inhibition in Pten mutant mice when the prepulse is comparatively quiet, as no impairments are reported for dB levels higher than 4 dB. Additionally, in accordance with our study, no differences in prepulse inhibition have been reported in the BTBR and Shank1 mouse models of autism14,15. This indicates that alterations in sensory reactivity may be a less sensitive measure of an autistic-like phenotype and may also only be present in particular ASD models.
Overall, the current study found that hyperactivity of the PI3K/AKT/mTOR pathway does not result in sensorimotor gating deficits in NS-Pten KO mice, suggesting that the pathway may not directly affect prepulse inhibition. This conclusion is supported by a prior study that assessed PPI in a transgenic mouse model of tuberous sclerosis complex, another model of ASD and mTOR hyperactivation, which similarly reported no deficits in prepulse inhibition between WT and KO mice16. Taken together, these studies indicate that despite mTOR’s contribution to an autistic-like phenotype, it does not significantly contribute to the onset of sensorimotor gating deficits in several different ASD models. Ultimately, our study is in support of the literature and helps to further elucidate the relationship between hyperactivation of the PI3K/AKT/mTOR pathway and deficits in sensory reactivity.
Figshare: Neuronal subset-specific Pten-deficient mice do not exhibit deficits in sensorimotor gating processes. https://doi.org/10.6084/m9.figshare.9885401.v113.
This project contains the following underlying data:
PPI Day1 Pten Raw Data 9-6.xlsx (raw data from all experiments performed for all animals; day 1).
PPI Day 2 Pten Raw Data 9-6.xlsx (raw data from all experiments performed for all animals; day 2).
PPI day 3 Pten Raw Data 9-6.xlsx (raw data from all experiments performed for all animals; day 3).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We would like to thank Samantha Hodges and Paige Womble for their critical review of the paper. The authors do not have any conflicts of interest to declare.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Prepulse inhibition, animal models of psychiatric disease.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Prepulse inhibition, Behavioural phenotyping of mutant mice, animal models of schizophrenia
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 4 (revision) 14 Jan 25 |
read | read | |
|
Version 3 (revision) 07 Apr 20 |
read | read | |
|
Version 2 (revision) 11 Feb 20 |
read | ||
|
Version 1 08 Oct 19 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)