Keywords
Immunochromatography kit, Norovirus, Rotavirus, Rapid detection, Bangladesh.
Immunochromatography kit, Norovirus, Rotavirus, Rapid detection, Bangladesh.
This version of the manuscript has the corrected tables with accurate sensitivity and specificity. Also some terms and statements are updated according to the reviewers' suggestions.
See the authors' detailed response to the review by Nicola A. Page
See the authors' detailed response to the review by Mustafizur Rahman
Diarrheal diseases represent a major worldwide public health problem, particularly in low-income countries1. Acute gastroenteritis is a very common disease in young children2. It has been reported that about 3–5 billion cases of acute gastroenteritis occur each year in children less than 5 years old and 1.5 to 2.5million children of that group die from severe diarrhoea3–5. Of that, about half a million death is caused by rotavirus infections6. On the other hand, norovirus is responsible for almost half of the foodborne gastroenteritis outbreaks and 75–90% of non-bacterial gastroenteritis outbreaks7.
When outbreaks of gastroenteritis occur in communities, rapid identification of pathogens is essential to ensure the administration of the appropriate treatment and control. Furthermore, definite diagnosis plays an important role to decrease the unnecessary use of antibiotics. In the case of emergency, there is no rapid detection method available in Bangladesh. In this regard, a rapid diagnosis kit with good sensitivity and specificity is essential. Developing such a kit may raise the reliability for rapid diagnosis in low-income countries, where the prevalence of norovirus and rotavirus is increased. Herein, we report a comprehensive analysis of the sensitivity and specificity of an immunochromatography kit (IC Kit) for the rapid detection of norovirus and rotavirus in Bangladesh.
In this study, we evaluate the newly developed IC test kit for norovirus and rotavirus detection (IP-Noro/Rota; ImmunoProbe Co., Ltd., Saitama, Japan) in 100 stool samples collected from paediatric patients with acute gastroenteritis (severe dehydration, abdominal pain and vomiting) in Bangladesh during January to June 2015. The study was ethically approved by the ethical review committee of Jahangirnagar University, Bangladesh.
Reverse transcription PCR (RT-PCR) was used as the reference test for both norovirus and rotavirus detection3. The PCR is a molecular biology technique that allows for nucleic acid fragment from a complex pool of DNA. Faecal specimens were thawed, diluted with distilled water to 10% suspensions, and centrifuged at 10,000xg for 10 min. Viral RNA was extracted from 140µl of the supernatant using a spin-column technique (QIAamp Viral RNA kit; Qiagen, Hilden, Germany) according to the manufacturer’s instructions. For reverse transcription, 3µl of extracted RNA was mixed with a reaction mixture consisting of 1µl of oligo dT primer (Promega, Madison, USA) and 1µl of nuclease free water in microcentrifuge tube, then kept at 70°C for 5 mins and then chill for 5 mins. After that 4µl of 5X reaction buffer (Promega, Madison, USA), 2µl of MgCl2, 1µl of PCR Nucleotide Mix (Promega, Madison, USA), 0.5µl of Ribonuclease Inhibitor (Promega, Madison, USA), 1µl of Reverse Transcriptase (Promega, Madison, USA), 6.5µl of nuclease free water were mixed with the same microcentrifuge tube. Then the solution was heated at 25°C for 5 mins, 42°C for 60 min and 70°C for 15 mins. Norovirus and rotavirus were detected by PCR analysis of cDNA with specific primers previously published8,9. The amplification was carried out in a thermal cycler (2720 Thermal Cycler, Applied Biosystems, USA). The PCR was performed at 94°C for 3 mins followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 60 s and a final extension at 72°C and then held at 4°C. The PCR products were electrophoresed in a 1.5% agarose gel, followed by staining with ethidium bromide (0.5 g/ml) for 20 min and then visualized under ultraviolet (UV) light. The bands were recorded by photography.
To evaluate the sensitivity and specificity of this IP-Noro/Rota test kits, all the 100 samples were tested for norovirus and rotavirus antigens by this kit following manufacturer’s instructions (IP-Noro/Rota; ImmunoProbe Co., Ltd., Saitama, Japan). It took only 10–15 min to obtain the result. A positive result for both pathogens is two lines; the left control line (C) and the right test-positive line (T), whereas, a negative result consisted of a single left control line (C) (Figure 1).
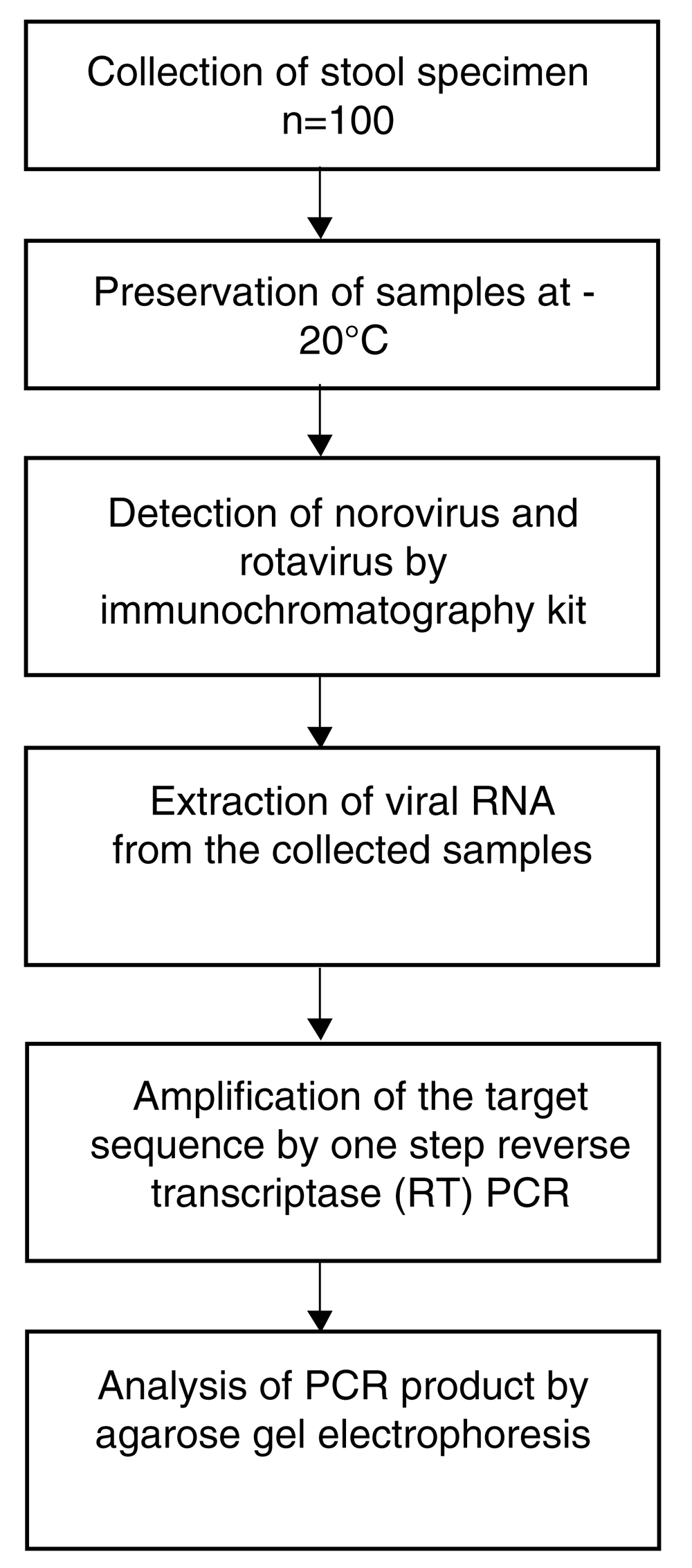
The working plan for evaluation of sensitivity and specificity of immunochromatography methods for rapid detection of rotavirus and norovirus associated with paediatric diarrhoea in Bangladesh is described in Figure 2.
By the RT-PCR method, 10 and 74 samples were confirmed as norovirus and rotavirus, respectively. It was found that all the isolated norovirus belongs to the genogroup II (data not shown). On the other hand, G1P8 rotavirus strain was found the most prevalent among the Bangladeshi paediatric population after characterization of G-types (VP-7) and P-types (VP-4) of rotavirus-positive samples. The youngest patient was 21 days and the oldest 56 months; the average age was 14 months. The most common clinical symptoms of rotavirus and norovirus infected patients were dehydration, vomiting, fever and abdominal pain.
Of the 10 and 74 samples positive for norovirus and rotavirus, respectively, by RT-PCR, IP- Noro/Rota kit recognized all positive samples with 100% sensitivity, which seem to be higher compared to other similar studies10,11. However, the kit might have given one false positives for norovirus and three false positives for rotavirus detection, resulting in a specificity of 98.9% and 88.5%, respectively (Table 1 and Table 2).
| Test Result | RT- PCR | Total | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| + | - | ||||
| IP-Noro/Rota kit: | 10 | 1 | 11 | 100% | 98.9% |
| + | |||||
| - | 0 | 89 | 89 | ||
| Total | 10 | 90 | 100 | ||
The clinical symptoms of the patients with acute gastroenteritis are generally not indicative of a specific pathogen. In Bangladesh, the outbreak of norovirus and rotavirus diarrhea occurs mainly in the winter season3, when the IC kits could be used for rapid screening, as other existing diagnosis methods are time consuming. The rapid IC kit test is easy to perform at a low cost and it takes only 10–15 min to diagnose with a simple procedure and does not require special equipment or a skilled technician.
Rotavirus and norovirus are the major enteropathogens responsible for acute viral gastroenteritis among infants and children in Bangladesh, where 74% of the cases were caused by rotavirus only. The IC kit provides a high specificity and sensitivity as well as good agreement with the reference method, RT-PCR, for the detection of rotavirus and norovirus. Therefore, IC-Noro/Rota kit will be easy and useful assay for the rapid detection of these viruses in routine diagnosis as well as during the outbreaks. This is the first report about the rapid detection of rotavirus by IC kits in Bangladesh. Finally, it is strongly recommended to use the IC kit as an alternative method for rapid diagnosis of norovirus and rotavirus infections, especially in low-income countries like Bangladesh.
Figshare: Raw Data- IC kit.csv, https://doi.org/10.6084/m9.figshare.7616630.v112.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank the ImmunoProbe Co., Ltd. (Saitama, Japan) for kindly providing the IP-Rota/Noro kit.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Virology and Molecular Biology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Viruses associated with diarrhoeal diseases, rotavirus vaccines, molecular characterization and phylogenetics of enteric viruses, epidemiology and public health.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Nguyen TA, Khamrin P, Takanashi S, Le Hoang P, et al.: Evaluation of immunochromatography tests for detection of rotavirus and norovirus among Vietnamese children with acute gastroenteritis and the emergence of a novel norovirus GII.4 variant.J Trop Pediatr. 2007; 53 (4): 264-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Viruses associated with diarrhoeal diseases, rotavirus vaccines, molecular characterization and phylogenetics of enteric viruses, epidemiology and public health
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Hossain ME, Rahman R, Ali SI, Islam MM, et al.: Epidemiologic and Genotypic distribution of Noroviruses among children with Acute Diarrhea and Healthy Controls in a Low-income Rural Setting.Clin Infect Dis. 2018. PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Virology; Infectious diseases; Rotavirus vaccine; norovirus epidemiology; viral hepatitis, HIV
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 02 Mar 20 |
read | read | |
|
Version 1 11 Feb 19 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)