Keywords
DNA barcoding, Kelabau Fish, Common Rivers of Riau, Population Structure
DNA barcoding, Kelabau Fish, Common Rivers of Riau, Population Structure
In this new version of the manuscript we have revised as follows:
1. We have added three citations in the discussion - "35,36" become "35 to 39".
2. In paragraph three in the discussion we have added reasons for limited variation and a discussion of data table 3.
3. Dr Windarti was added as a co-author for her work in improving the manuscript
See the authors' detailed response to the review by Ja’afar Nuhu Ja’afar
See the authors' detailed response to the review by Indra Junaidi Zakaria
See the authors' detailed response to the review by Jayasankar Pallipuram
Kelabau are ancient fish belonging to genus Osteochilus of family Cyprinidaes. Kelabau fish are distributed throughout Thailand, Vietnam, Peninsular Malaysia, Borneo and Sumatra1,2. In Sumatra Island in Indonesia, these fish is commonly found in the Siak, Kampar and Rokan rivers in Riau Province3,4.
According to local fishers in Riau, kelabau are divided into two types on the basis of morphology; although, there is no detailed information about these fish types. The local fishers said that the first type of kelabau fish is the fish found in this study. The morphological characteristic of this fish is brownish body, with brighter bottom, dark hazy blotches present above the pectoral fins, and the size ranges from 15 to 2,778.93 gr. The second type of fish is larger sized and yellowish color. However, during the study time, from 2017 to 2018, this type was not found. Thus, a study was needed to identify the species using morphological and molecular methods to determine these types in the Siak, Rokan and Kampar rivers. Identification of any species using morphological traits can be difficult and can lead to errors5; therefore, owing to morphological similarities among Osteochilus spp., molecular markers, such as DNA barcodes, are important to identify the fish species uses a specific sequence region (i.e. cytochrome c oxidase subunit 1 (CO1)) to identify a species and is a technique that can identify taxonomic units as well as biodiversity for determining species of several organisms5–9. Unlike molecular phylogeny used to determine relationships among species, the purpose of DNA barcoding is to identify unknown or undetermined species into phylogeny10. The common mitochondrial (mt) DNA region used as a barcode in protists and animals comprises 600 bp. In addition, CO1 is one of the genetic markers used to identify insects, birds, primates and fish to species11–13. MtDNA CO1 is selected as a target in DNA barcoding because it is a highly conserved site. This method has advantages over the morphological identification approach in that it is fast, reliable and it can be used for all types of samples because it uses a single gene along with mutations in the nucleotides to acknowledge the taxonomic features of each species13.
The study on DNA barcoding for freshwater fish has been widely practiced in various countries, including Nigeria14, India15, Philippines16, Canada9 and Indonesia5,17–19. The method has been successfully validated for the taxonomic status within Rasbora in Lake Laut Tawar20; Anguillidae in Aceh waters21; Ornamental fish from Peat lands5; Channidae in Peninsular Malaysia, Sarawak, Sumatera, Borneo, Myanmar, Vietnam, India, Germany, Singapore and the United Kingdom22 and Cichlidae in northeastern Nigeria23; therefore, it can be used to equally successfully validate the taxonomic unit of the kelabau using its morphology supported by molecular data. This information is crucial for designing a remedial course of action about the conservation strategy for this species in the Siak, Kampar and Rokan rivers in Riau Province, Indonesia.
The study population was collected and sampled according to the guidelines on the use of living organisms for research from the Laboratory of the Faculty of Fisheries and Marine, Riau University, Indonesia24
A total of 90 kelabau (30 fish from each river) were collected from the Siak, Kampar and Rokan rivers (Figure 1). Fish were caught using a gill net 3 m deep and 20 m long with a 12.7-cm mesh. The gill nets were installed in the river water close to the riverbank and remained for 24 h from 08:00 to 08:00 the following day. The fish collected were counted using hand-counter and cleaned using freshwater. A number of 50-mm caudal fin tissue samples were taken using a sterile scissors and preserved in ethanol, after which a photo of each fish sample was taken for documentation using a digital camera.
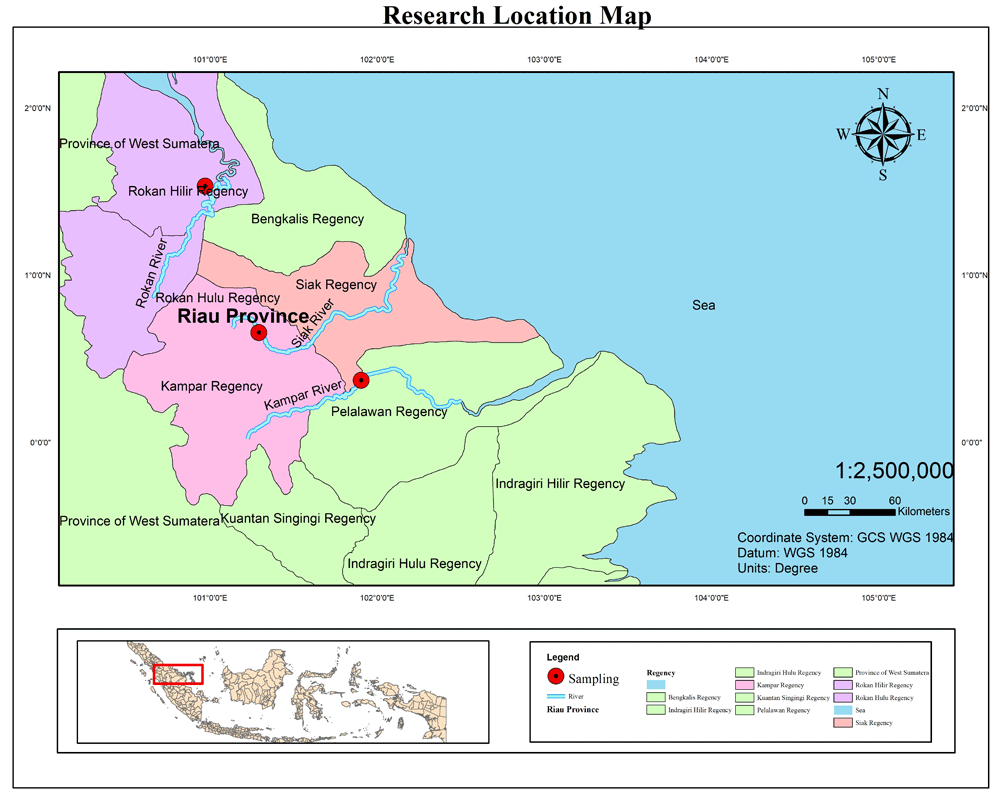
All samples were preserved in 3-kg sample bags which were labeled according to site location, date and serial number. Before preservation, the fish samples were injected with 10% formalin. The fish samples were then transported to the laboratory for further evaluation. The morphologies of the collected fish were identified up to species level using the identification book produced by the Indonesian Institute of Sciences ichthyology museum1,25. The fish morphologies observed were length, color, shape of scales, mouth shape, barbels, number of fins and special marks on the body.
Biometric analyses were used to measure morphological characteristics in this study1. This tool is considered conventional for identifying organisms. Molecular identification using CO1 gene sequences has been supported for providing additional organism classification.
DNA was extracted using the spin-column method from the gSYNC DNA Extrusion Kit (Geneaid Catalogue No. GS 300, Taiwan). The extracted DNA was then transferred to a 1X Tris–borate ethylenediaminetetraacetic acid (TBE) solution with a 1.5% agarose gel and Pegreen gel dye (PEQLAB Biotechnologies GmbH, Erlangen, Germany)19. The quantity of DNA was visualized with the help of a GeneQuant Spectrophotometer by adding 78 μL nuclease-free water in a cuvette along with 2 μL DNA. The DNA was then amplified using the universal primer Fish-F1(5’-TCA-ACC-AAC-CAC-AAA-GAC-ATT-GGC-AC-3’) and Fish-R1 (5’-TAG-ACT-TCT-GGG-TGG-CCA-AAG-AAT-CA-3’) with a target of 707 bp and 655 bp26, respectively. The amplification thermocycling conditions as follow: the PCR condition using pra PCR (94°C for 5 min), 35 cycles of denaturation (94°C for 30 s), annealing (56.6°C for 30 s) and extension (72°C for 30 s), followed by post-PCR extension (72°C for 5 min) and hold (4°C for 5 min). PCR results were analyzed using 1.5% agarose gel at 100 V to assess the bands, and only the clear products were sent to Applied Biosystems Macrogen Korea for sequencing.
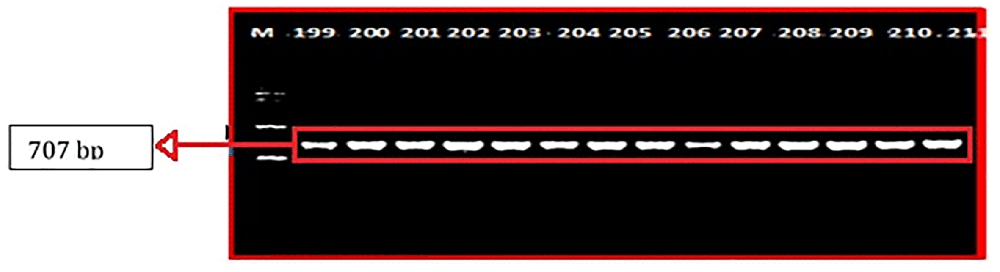
The PCR amplicon was 707 bp, which implied that no sequence of DNA was derived from mtDNA nuclear mitochondrial DNA segments (NUMTs), because a NUMT barely reaches 600 bp9. The selected CO1 sequences were entered into GenBank and the BOLD System databases to compare the alignment of nucleotide sequences and 99%–100% values with that with no insertions/deletions. All sequences were aligned using ClaustalW with MEGA v.727.
The entire nucleotide sequence obtained from the sequence chromatogram was assembled using DNA Baser Assembler, aligned and then analyzed using MEGA 7. It was further aligned (multiple alignments) using the reference NCBI GenBank accession numbers AP011385.1 and KC631202.1. Similarly, the percentages of CO1 sequences were blasted using NCBI Blast and BOLD Systems databases.
Nucleotide variations among samples were analyzed using dnaSP v.528. The parameters of these calculations were haplotype number, variable site, parsimony site, haplotype diversity and nucleotide diversity.
Phylogenetic trees were estimated using all samples from the three populations and calculated according to the Tamura-Nei model29 using MEGA 727.
The distance among the nucleotide bases of the mtDNA CO1s was analyzed using the Kimura 2-parameter model30. The nucleotide distances between and within the populations were examined according to the model based on the similarity of frequencies and ratios of transition to transversion (Ti:Tv) using MEGA 727.
The morphological traits of all kelabau used in this study matched those of O. melanopleurus. We used the important morphological traits to identify these fish according to Kottelat et al.1. The morphological characteristics measurement of O. melanopleurus showed that the fish have 16–19 branched dorsal rays, the number of scales was ranged from 10.5 to 12.5 in between dorsal origin and lateral line, the number of circum peduncu-lar rows of scale was ranged from 20 to 24 and lips covered with folds and plicae and there was no hard tubule at the tip of the mouth (Figure 2a). This species has one pair of barbels at above and one pair at bottom, dark hazy blotches near above of the pectoral fins. The body is brownish, with the bottom brighter than the top and the type of steroid scales (Figure 2b). Raw biometric data are available on OSF31. as ~707 bp.
A sequence amplified by Fish-F1 primer was successfully identified in 86 of 90 samples of mtDNA fish. The base length of the CO1 nucleotide obtained from the formulation process and electrophoresis (Figure 3)
Based on genetic analysis using the Tamura-Nei model, there was an unequal distribution of all nucleotides with the following frequencies: adenine (A), 26.73%; thymine (T), 30.44%; cytosine (C), 25.93% and guanine (G), 16.90% (Table 1). The rates ratio between transition and transversion was 10.257 purines and 1.915 pyrimidines, and the overall transition and transversion bias were R= 2.499 based on Tamura-Nei model32. The pattern of nucleotide distribution of A, T, C and G was T > A > C > G, but the carps which cultivated in India have the following pattern of nucleotide distribution: T > C > A > G33.
| A | T | C | G | |
|---|---|---|---|---|
| A | - | 4.03 | 3.34 | 22.95 |
| T | 3.54 | - | 6.57 | 2.24 |
| C | 3.54 | 7.72 | - | 2.24 |
| G | 36.29 | 4.03 | 3.34 | - |
The nucleotide distances between nucleotide bases within the groups indicated that the values of the nucleotide base sequences within the fish population were 0.0009, 0.0000 and 0.0010 in the Siak, Kampar and Rokan rivers, respectively. The evolutionary distance between the nucleotides of the groups had a comparative difference in the nucleotide sequences of 0.0005 for fish from the Siak and Kampar rivers, of 0.0009 in those from the Siak and Rokan rivers and of 0.0005 for those from the Rokan and Kampar rivers. Based on the nucleotide distance, the fish were identified as being from the same species (0.06%) (Table 2).
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Siak | - | |||
| Kampar | 0.0005 | - | ||
| Rokan | 0.0009 | 0.0005 | - | |
| GenBank (AP011385.1) | 0.0367 | 0.0366 | 0.0368 | - |
| GenBank (KC631202.1) | 0.0351 | 0.0350 | 0.0351 | 0.0048 |
i Source: Kimura estimation of 1980: “Evolutionary Divergence over Sequence Pair Between Groups”30.
CO1 had 612 conserved sites (98%), 9 variable sites (1.45%), 4 informative parsimony (0.64%) and 5 singleton sites (0.81%). The highest nucleotide variation was in the Rokan river population (0.00100 ± 0.00032); whereas, the Kampar river population had no nucleotide variation based on DnaSP5 calculations (Table 3). Using the NCBI database with accession numbers AP011385.1 and KC631202.1, the DNA sequence of Kelabau was identified as belonging to O. melanopleurus with 96%–97% accuracy, query coverage of 99%–100% and an E-value of 0.0. While based on the BOLD System, the identity of all samples was 96.60%–96.93% accurate.
i Source: Nei, 1987 for haplotype and nucleotide diversity34.
In the phylogenetic tree consisted of two major groups (Figure 4). The first group was Cirrhinus moltonela (Gen-Bank KC631192.1) and it was divided from O. melanopleurus. The second group was differentiated into two sub groups, O. melanopleurus from GenBank (AP011385.1 and C631202.1) and 86 fish samples from Kampar, Siak and Rokan rivers. The 86 samples have BLASTN similarity values with O. melanopleurus of 96%–97%.
Overall, the morphological traits and DNA barcoding showed that the majority of, if not all, kelabau fish in the three rivers at Riau Province were O. melanopleurus. there were 2 types of kelabau fish present in Riau. The first type is relatively small (15 gr to 2,778.93 gr) fish with dark colored dorsal and whitish ventral. This type of fish is used in this study. The second type of kelabau fish is larger, with yellowish color. This fish was not found during the study.
Environmental changes can cause fish death orbmigration to suitable habitats. Overfishing using both legal and illegal methods has also triggered the decline of certain species35–39. In addition, our results suggested that there was little nucleotide diversity among O. melanopleurus in the Siak, Rokan and Kampar rivers in Riau Province, particularly the fish in the Kampar river
The lack nucleotide diversity of O. melanopleurus from the three rivers was likely to be caused by limited opportunities for kelabau migration, so that the genetic exchanges with other populations are very small36; moreover, the lack nucleotide diversity is believed to be caused by inbreeding, and overfishing5,40. Variation in nucleotide diversity of the fish from the sampling areas is different. In the Kampar River, the fish was sampled from the relatively narrow area and it may cause the lowness of the nucleotide diversity. The diversity was 0 and it means that the nucleotide of the fish samples was almost identic. The low nucleotide diversity may occur as the habitat of the fish is very suitable and the fish may not face environmental related problem that trigger any genetic changing41. However the nucleotid diversity of the fish from the Siak and Rokan River was higher than Kampar River. Siak and Rokan Rivers have relatively low water quality that was caused mainly by anthropogenic activities. Changing in water quality may trigger the fish to adapt with that environmental condition and it may cause changes in genetic variation. Freeland et al.41 stated that fish population with high genetic variation may be able to face problems related to environmental changing
In addition, Kelabau from the Siak and Rokan rivers were designated as one sub-sub group in group two (Figure 4) because both rivers are geographically connected, allowing for hybridisation, whereas, there is inbreeding of these fish in the Kampar River, which causes a nucleotide diversity value of 0.0. Geographically, these three rivers (Siak, Kampar and Rokan rivers) have different environmental condition, that cause variations in morphology in fish (O. melanopleurus) based on truss morphometric42. Factors causing morphological variations are environmental differences, habitat pollution levels, long-term isolation and crossbreeding in populations43,44. The same deductions were drawn from previous studies on Desmopuntius pentazona and D. rombochelatus, although the taxonomies of the two fish are different. Nevertheless, based on a genetic difference of only 0.4%, the two species were grouped into one cluster5. These are distributed throughout Asia, the Mekong and Chao Praya river basins, Peninsular Malaysia, Sumatra and Borneo1,2.
The identity of a species was derived using the morphological identification method to distinguish between species or individuals45,46. Basically, the genetic identification of a species can be done using mtDNA CO1, a more effective approach than using rRNA5,33. The nucleotide locus and mutations were used as references to conduct DNA barcoding in all fish samples13. Previous studies have identified several species using DNA bar-coding, such as ornamental fish of wetlands5, wetland fish larvae46, rainbow fish47, Cyprinidae fish48, salmon and trout7 and freshwater fish9,19. Furthermore, the phylogenetics of CO1 sequences can effectively show congeneric and confamilial species9.
The phylogenetic trees could describe the line of biological evolution from species or organisms with a different ancestry49. Nonetheless, the results of all these species did not show a 100% undistinguishable identity. The branch length between species leading to a gap in the pairwise distance distribution is referred to as the barcoding gap in CO123. Intra-species relationships were quite high in all samples, which confirmed that kelabau melanopleurus) were native in the three rivers and could to adapt to changes in environmental conditions40.
Moreover, the existence of inter-nucleotide patterns and distances between A, T, C and G in the chromosomes showed the characteristics and genetic signs that distinguished each of the individuals, even though they belong to the same species50. This is reinforced by referencing the phylogenetic tree made using the neighbor-joining model.
The identification of fish species is normally conducted using morphological characteristics such as dorsal fins, pelvic fins, pectoral fins, anal fins, linea lateral fins, upper linea lateral fins, lower linea lateral fins, around body fins, and caudal peduncle fins; however, in this study, we used 12 morphological traits as described by the classification system of Kottelat et al.1. These results supported the classification using biometric data that all fish in the three rivers were O. melanopleurus. The morphological characteristics were consistent with the species having a relatively large body with a standard length of 119–560 mm, lips covered with folds and plicae, no tubercles on the snout, a pair of maxillary barbels, and a pair of lower jaw barbels. The body is brownish, with the bottom brighter than the top. Dark hazy blotches near above of the pectoral fins, which is a special trait of O. melanopleurus.
However, this method can be difficult, and molecular identification is necessary. In particular, using mtDNA CO1 was an effective approach9,17. The results from nucleotide distance data based on the Kimura 2-parameter model indicated that the nucleotide distance among the fish was short in intraspecific species using mtDNA CO140, which was supported by data showing that the percentage identity in O. melanopleurus species ranged between 96% and 97%. The Kelabau fish from the three sample sites were identified as O. melanopleurus by percentage identity, supported by an E-value of 0.0 and a 99%–100% query cover. The p-value indicated that the BLASTN results contained no errors. Besides, the low nucleotide distance values (<3%–5%) among the samples of O. melanopleurus from the Siak, Kampar and Rokan rivers, indicated that all samples were monophyletic.
Based on our findings, we concluded that 86 of the 90 samples of kelabau from the Siak, Kampar and Rokan rivers in Riau were O. melanopleurus, as revealed by their morphological traits and the molecular analyses.
CO1 gene sequences and raw biometric data of Osteochilus melanopleurus from Riau rivers can be found on OSF.
CO1 gene sequence DOI: https://doi.org/10.17605/OSF.IO/XGEZD51
Raw biometric data DOI: https://doi.org/10.17605/OSF.IO/CFGM831.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The raw CO1 sequence data were also deposited in GenBank and can be found under sequential accession numbers MH430827-MH430854 and MH459085-MH459142.
The authors thank Neli Safrina as a staff Laboratory of Fisheries and Marine Faculty, Riau University and Gema Wahyu Dewantoro, as a staff researcher at the Laboratory of Ichtiology in the Field of Zoology, Research Center for Biology of the Indonesian Institute of Sciences (LIPI) on the identification of the Kelabau fish using morphological traits. Also, thanks to Ornamental Fish Aquaculture Research and Development Center, Ministry of Marine and Fisheries Republic of Indonesia, Depok for providing laboratory facilities.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fish Biology and Fisheries biology
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular diagnostics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I have been working on molecular taxonomy of fish, shellfish and marine mammals since 1996, thus 23 years of experience in this field; also gained expertise in freshwater aquaculture technology during the past 11 years.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular diagnostics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fish Biology and Fisheries biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 10 Mar 20 |
read | ||
|
Version 2 (revision) 23 Jan 20 |
read | read | read |
|
Version 1 12 Feb 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)