Keywords
Head and neck cancer, Oral mucositis, Concurrent chemo-irradiation
Head and neck cancer, Oral mucositis, Concurrent chemo-irradiation
Globally, head and neck cancer (HNC) accounts for 550,000 cases and 380,000 deaths, annually1. In the US, HNC accounts for 3% of cancers2, and in Europe, it was estimated to be 4% in 20123. Men are affected more than women with a ratio of 2:1 and 4:1 for US and Europe, respectively. In Iraq, the incidence of HNC is <2%4. The primary causes of HNC are tobacco and alcohol, and viral infections including Epstein-Barr virus and hepatitis virus5–7.
Radiotherapy (RT) plays a central and evolving role in the treatment of HNC8 and is used with or without chemotherapy (CT) as a definitive or adjuvant treatment8. The oral cavity is susceptible to direct and indirect toxic effects of cancer CT and RT9. This risk reflects high rates of cellular turnover for the lining mucosa, a diverse and complex micro-flora, and trauma to oral tissues during normal oral function10–12. Oral mucositis (OM) is a debilitating side effect of RT13 and is exacerbated by concomitant CT14, which can begin 1–2 weeks after initiation of RT as asymptomatic erythema often progressing to erosion and ulceration. The ulcers are painful, covered by a white fibrinous pseudo-membrane, associated with dysphagia and decreased oral intake15,16.
Radiation therapy is an important method used in the treatment of head and neck cancers and, like all other methods used in treatment, it is not without the side effects of treatment. The injuries that occur to mucous membranes of the oral cavity are only part of those effects, which we can avoid and reduce them before and during and after treatment by adhering to the recommendations of the treating physician and the work of therapeutic planning. This study aimed to identify the incidence, distribution of OM, and its effect on treatment breaks in a section of HNC in patients in Iraq.
This is an observational, cross-sectional study for HNC treated by external beam radiation therapy (EBRT), which included patients 50 patients, who were patients from 30th April to 10th September 2017.
The study was conducted at Baghdad radiation Oncology and Nuclear Medicine center, Bagdad, Iraq.
Patients who fit the eligibility criteria during the study dates who were scheduled for treatment were included in the study.
Dose of EBRT: 50-70 Gray, with a standard fractionation. Each fraction is 2Gy and 5 fractions per week. Radiation delivered with 3D conformal technique, using Elekta infinity, and Elekta synergy machines.
Eligibility criteria of patients was: primary HNC; T3 or T4 disease; positive nodes; residual disease; positive margins; perineural invasion; lymphovascular infiltration; extracapsular extension; treatment as described above.
Patients were excluded who had comorbid conditions, were treated in a palliative way, and those with metastasis or with a bad performance status.
We conditionally collected data from patient files when they attended follow-up at the in-patient or/and out-patient clinic, or when these patients made visits to our center.
Variables collected:
Variables collected included: patient’s gender, age and smoking status; tumor histopathology, stage, grade, subsites, and primary or metastases; radiotherapy dose, fractions, interpretations and oral mucositis onset.
Assessment by World Health Organization’s scale of OM was performed as follows: Grade 0, no OM; Grade I, soreness; Grade II, erythema, ulcers, able to eat solids; Grade III, Ulcers, liquid diet only; Grade IV, alimentation not possible.
The collected data was categorized and analyzed by T-test to identify the incidence of OM and its distribution. SPSS IBM version 22 was used.
Written informed consent was obtained from the patients for the publication of their data in this article, and the study was conducted according to the ethical standards established by the 1964 Declaration of Helsinki. The Medical Ethical Committee of Baghdad University approved this study (code:611) on 18/04/2017.
In the total patient population 72% were men, while 28% were women; male/female ratio was 2.5:1. The mean age was 53.3±11 years, and majority (38%) were between the ages of 55 and 64 years. Patients aged 65 years or more composed 20% of the total population (Table 1). In total, 76% received CT before or concurrent with RT. The vast majority (86%) had advanced stages III or IV of cancer. 74% were smokers, during or before starting RT (Table 1); 86% of these were men, while 43% were women. Seven sub-sites were observed. The highest was the nasopharynx, followed by the larynx, parotid, and then the tongue (Figure 1).

In total 72% of patients had an incidence of OM, with no patients with grade IV; 40% were grade II, and grades I and III appeared in 16% of patients (Figure 2). OM occurred in 100% of young patients, below 35 years, 50% among 45–54 years old, and 90% in patients ≥ 65 years. 93% of women developed OM compared to 64% of men (Table 2). OM appeared in 50% of patients treated with RT only, and 79% of those treated with concurrent protocols (Table 2). Grade III OM occurred in 18% of patients treated with RT and CT, while it occurred in 8% with RT only. OM appeared in 42% of patients with stage II cancer, and 77% and 75% of those with stages III and IV. OM occurred among 73% smokers and 69% nonsmokers. But severe OM (≥ grade III) occurred in 26% of smokers and 11% of non-smokers (Table 2). In this study, the majority of OM cases (47%) came from nasopharynx tumors (Table 2).
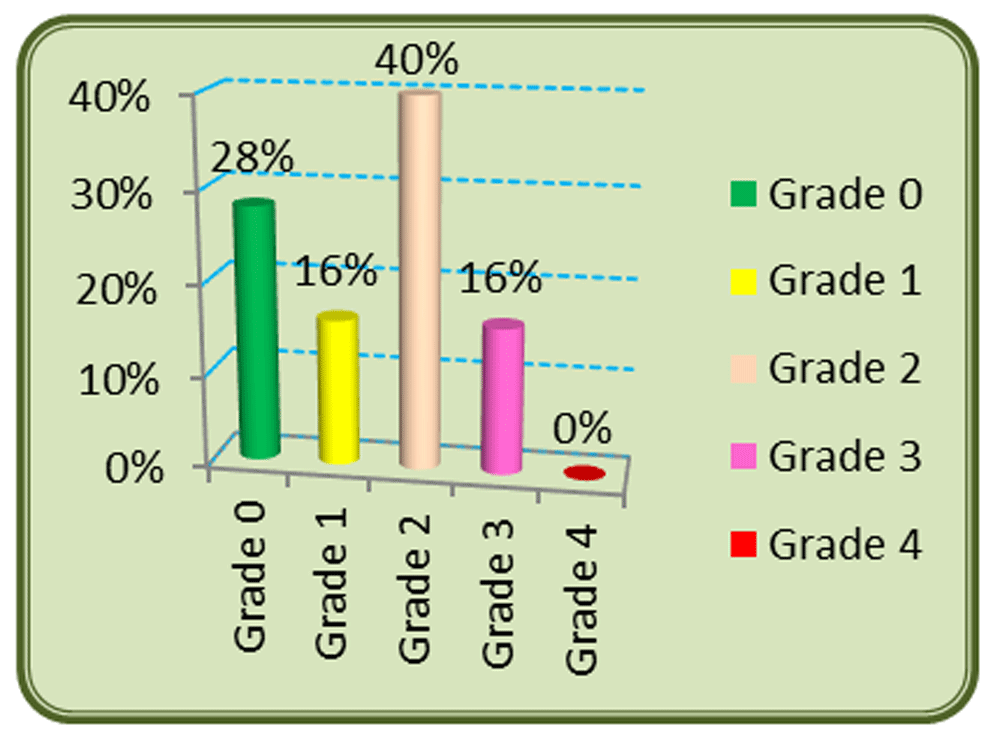
RT, radiotherapy; CT, chemotherapy.
In total, 20% of patients had single breaks in their treatment schedule; the total break days was 20 days, giving an average of 2 days per break (Figure 3). Unplanned breaks were observed more in men, those who smoke, those with both RT and CT treatment, those with stage IV cancer, and those with grade III OM (Table 3).

RT, radiotherapy; CT, chemotherapy.
In most countries around the world, HNC is most common in men, and the male/female ratio ranges from 2:1 to 4:17,17; in the UK, the ratio of male/female was 2.7:1, and in Australia it was 2.6:118,19. In our study the ratio was 2.6:1, which is consistent with studies elsewhere. In a similar study, Vera et al. found the mean age of 450 HNC patients was 61.3 ±12.3 years14, and it was 53.3 ±11 years in our study. According to Cancer Research UK, 50% of HNC cases each year are diagnosed in people aged 65 and over18. Some of our patients were aged 65 years but only constituted to 20% of the study population. This difference in age incidence can be attributed to many causes, such as the rising age incidence of HNC in the West17, higher life expectancy in those countries20, differences in environment, and differences in life style21.
In the current study OM occurred in 72% of patients, while in other studies OM occurred among virtually all patients who are undergoing RT11,21. Eilers and Million consider being female, young age and elderly as risk factors for developing OM22. This agrees with our study; 93% of females had OM, and there were high rates observed in the age groups of <35 years and >65 years.
In our study, grade III OM occurred in 18% treated with both therapies, while its appearance in 8% only among those who received RT only. Lalla et al. found a high incidence of OM with primary tumors in the oral cavity, oropharynx or in the nasopharynx16. This is well observed in the current study in which all patients had primary tumors. All patients with oral cavity, tongue, maxillary sinus and post-cricoid tumor developed OM. 94% of patients with nasopharynx tumor had OM and 47% were seen in nasopharynx patients. The tongue, oral cavity, and maxillary tumors constituted 36% of OM, while parotid tumor contribution was 11%.
The main principles of treatment by radiation is to deliver the total fractionated dose without interruptions. However, in daily clinical practice unplanned treatment interruptions are inevitable. Bese et al. concluded that patients, because of moderate or severe ulcerative OM had 15.8% and 46.8% incidences of RT break, respectively23. In our study, among 36 patients with OM, 10 of them (27.8%) needed breaks. The percent of breaks was high in males (35%), smokers (30%), those who were treated with CT plus RT (30%), those with stage IV (44%), and those with grade III OM (50%). In this study, two patients had breaks before the end of the 3rd week, and four had unplanned breaks at the 6th and 7th weeks, so a total of six patients (12%) had breaks at a critical treatment time. However, the break durations were only 1–3 days, but it is important to mention that in most of the times, the break is decided by patients themselves (subjective). It is convenient to mention here the conclusion of Bonomi et al., that OM is not only painful but also decreases the patient’s willingness to continue treatment24.
OM is an ongoing toxicity of RT, yet it still represents an important clinical challenge and causes burden to patients and caregivers. Most patients with HNC treated by radiation develop OM. The sub-site of the tumor is a main risk for development of OM. It was observed that young and old ages, combined RT plus CT, and advanced stage of tumor are associated with high incidence and severe OM. Patients with OM are at high risk of unplanned breaks in radiation. HNC in Iraq attack young and middle age people, which may lead to increases on its social and economic burden.
1. Using a multidisciplinary approach for oral management of HNC, before, during and after treatment.
2. Provision of psychological care and support services for these types of patients.
3. Education of patients and families regarding oral care.
4. Encouragement and support of multi-center studies and researches.
5. Raising competency of dentists, primary health care physicians and dermatologists to ensure early detection of HNC.
Zenodo: Excel sheet file of 50 head and neck cancers whom suffer from oral mucositis due to radiotherapy, http://doi.org/10.5281/zenodo.254320425.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, Statistics, Cancer Registries Data
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Toro J, Arango J, Westbrook S, Jewell P, et al.: Oral Mucositis Measured by the Oral Mucositis Assessment Scale (OMAS), but Not by the World Health Organization (WHO) Scoring System, Correlates with Nutritional Changes after Autologous Peripheral Blood Stem Cell Transplantation.Blood. 2007; 110 (11). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pathology, Oncoloy and Genomic Medicine
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Franco P, Martini S, Di Muzio J, Cavallin C, et al.: Prospective assessment of oral mucositis and its impact on quality of life and patient-reported outcomes during radiotherapy for head and neck cancer.Med Oncol. 2017; 34 (5): 81 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Radiation oncology; head and neck cancer; clinical oncology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 14 Feb 19 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)