Keywords
Levetiracetam, anti-epileptics, hepatotoxicity
Levetiracetam, anti-epileptics, hepatotoxicity
Seizure complicates major subsets of patients with stroke, and newer anti-epileptics are being favored in many clinical settings for seizure prophylaxis due to their good safety profile1. Levetiracetam has become one of the most commonly used antiepileptic in current practice for treatment as well as prophylaxis against seizures.
Drug-induced liver injury owing to antiepileptic drugs (AED) is well recognized2. It has been reported to occur more commonly with phenytoin and carbamazepine, and very rarely with valproate2. The consequences of such an injury can be alarming, resulting in harbingering death or the need for liver transplantation. Therefore, newer AED with minimal or no hepatic metabolism are being favored as first line drugs3.
Herein, we report a case of acute liver injury following levetiracetam usage in a post-operative patient of intracerebellar hemorrhage at our Neurosurgery Intensive Care Unit. We implicate a rare but life-threatening effect of a very commonly used anti-epileptic drug. There have been only few case reports of acute liver failure following use of levetiracetam and none were in post-operative neurosurgery cases4–6.
A 55-year-old male patient from a remote village in Biratnagar presented to our emergency department with complaints of sudden onset dizziness, slurring of speech and headache. He was a known hypertensive but not on regular medication or regular follow-up. Neurological examination revealed Glasgow Coma Scale (GCS) of eye opening 4; Verbal 5; and Motor 5, on admission with his bilateral pupils equal and reactive to light. He had no focal neurological deficits or features of meningeal irritation. An emergent Computerized Tomography scan of the head showed features suggestive of cerebellar bleed with fourth ventricle compression with herniation and ventricular extension. While arranging for cerebral angiography, there was a sudden fall in GCS to E1V1M3, and thereby the patient underwent emergency suboccipital craniectomy with evacuation of cerebellar bleed with placement of external ventricular drain.
The patient’s post-operative medications included ceftriaxone (1gram intravenous every 8th hourly), prophylactic levetiracetam (500 milligram intravenous every 12th hourly), Pantoprazole (40 milligram intravenous every 12th hourly), amlodipine (5 milligram via nasogastric tube 12 hourly), Losartan (50 milligram 12 hourly via Nasogastric tube), and Metoprolol (50 milligram via nasogastric tube 12 hourly). His immediate post-operative GCS improved to E3VtM6.
However, on 3rd post-operative day, the GCS fell to E1VTM4. Repeat CT head was uneventful. The patient was noted to have gross icterus and his liver function test revealed total bilirubin of 9.4 mg/dl (normal, 0.1mg/dl), direct 2.0 mg/dl (normal, 0-0.35mg/dl); aspartate aminotransaminase/serum glutamic-oxaloacetic transaminase (AST/SGOT) of 911 IU/L; (normal, 10–40 IU/L); alanine aminotransferase/serum glutamic-pyruvic transaminase (ALT/SGPT)of 926 IU/L (normal, 10–40 IU/L); alkaline phosphatase (ALP) of 298; (normal, 40–112 U/L); International Normalized ratio (INR) of 1.09 (normal, <1.1). Complete blood counts were done to rule out sepsis and were normal. Ultrasound of the abdomen and peripheral smear (for identifying features of obstructive jaundice as well as portal hypertension and ruling out hemolysis for raised bilirubin respectively) were normal. So, a possibility of drug induced liver injury causing acute hepatic failure was considered. Since none of the drugs prescribed were commonly implicated to have hepatotoxic effects, we considered the possibility of levetiracetam following a thorough literature search and hence stopped the drug. We also prescribed prophylactic hepatic encephalopathy regimen with strict monitoring of urine output, GCS, watching for seizures and features of upper gastrointestinal bleed. From the second day of stoppage of the drug, repeat laboratory tests showed gradual improvement in liver functions (Figure 1 and Figure 2) paralleling clinical improvement.
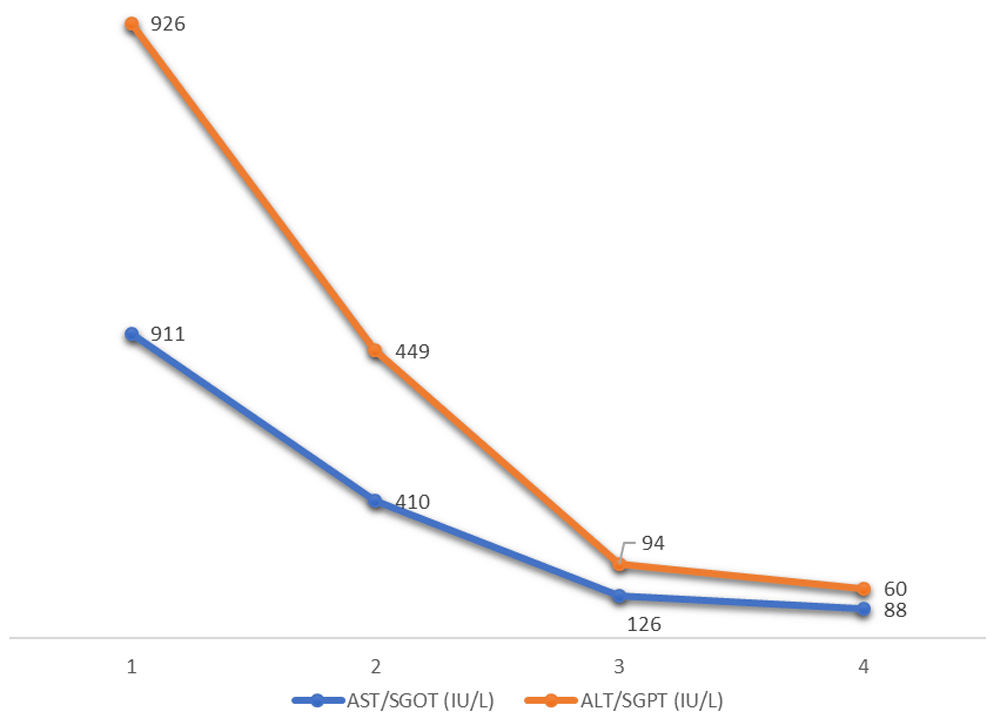
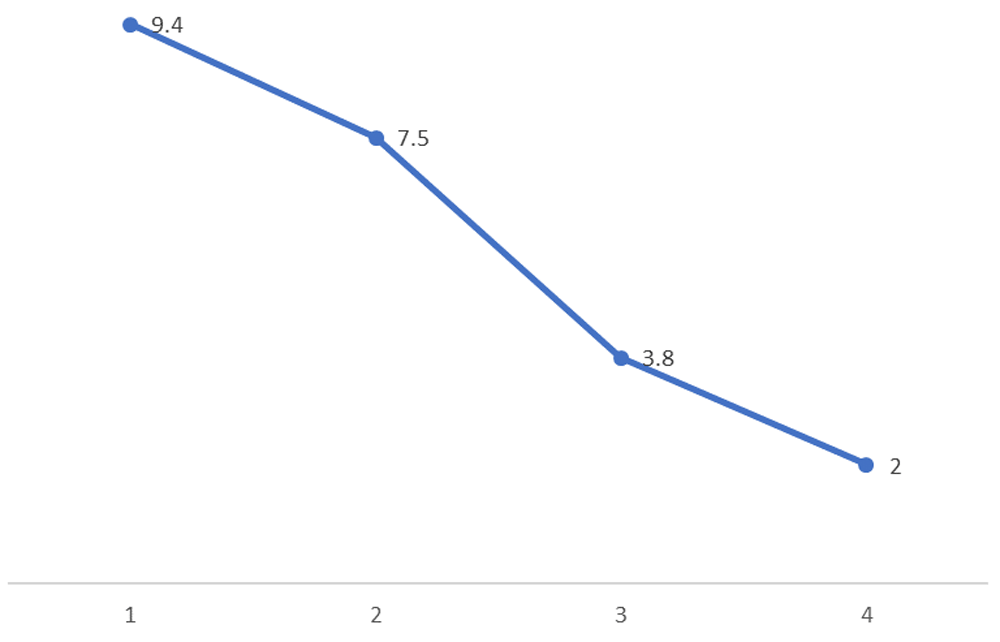
Although restarting the patient with the same drug and aided with liver biopsy would be more diagnostic, in our case, the patient’s hepatic function rapidly normalized following stoppage of only levetiracetam from our prescribed drug lists. Therefore, we sufficiently concluded that levetiracetam caused the hepatotoxicity. Though rare, we would like to stress upon the importance of keeping this rare but life-threatening complications of levetiracetam in mind, as it can have profound effect on the timely and corrective management of the patient. There was no episode of recurrence of jaundice seen in the patient within the ensuing 3 weeks.
Seizures complicate up to 20% of cases with spontaneous intracerebral hemorrhage7. There is no high level of evidence favoring the use of a specific AED7. Levetiracetam is one of the most commonly used AED in current clinical practice due to its relatively good drug safety profile, and most adverse effects mentioned are usually mild to moderate in intensity8. Levetiracetam does not inhibit or induce hepatic enzymes and most of it is eliminated unchanged by the kidneys. Thus, because it is minimally protein bound and lacks metabolism by the liver, the risk of hepatotoxicity is low. Thus, levetiracetam has a wide safety margin8.
However, while reviewing literature, we found a few case reports citing hepatotoxicity with levetiracetam usage4–6. As per the current recommendations by the Council for International Organization of Medical Sciences (CIOMS) for diagnosing drug induced liver injury, our case was of hepatocellular variant9. However, there is no consensus or diagnostic modality in correctly determining the implicated drugs, and thereby this has to be relied solely on the basis of empirical decision as to discontinue or modify drugs during such a scenario9.
Tan et al. reported incidence of fulminant liver failure owing to levetiracetam4. Syed and Adams also reported a case of liver failure following prophylactic levetiracetam usage in a patient with head injury5. Sethi et al. reported a post-traumatic head injury patient who developed asymptomatic elevation of hepatic enzymes following levetiracetam usage6.
Though safe and free of major side effects when comparing to older AED, it is however prudent to note that there are reports of liver injury following levetiracetam, ranging from asymptomatic elevation of transaminases to fulminant hepatic failure. Though routine liver or renal function monitoring may not be needed, it is advisable to keep the patient informed of such possible side effects with the use of newer AED like levetiracetam.
Since the patient was not fully conscious and alert enough to understand the concept of signing consent (since he was in a rehabilitation phase following intracranial hemorrhage), written informed consent for the publication of the relevant clinical and radiological data was obtained from the patient’s wife.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacovigilance, pharmacoepidemiology
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacovigilance
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Feb 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)