Keywords
hidradenitis suppurativa, psoriasis, certolizumab, brodalumab
hidradenitis suppurativa, psoriasis, certolizumab, brodalumab
In the revised version of our manuscript, we changed the title to “Treating co-existence of hidradenitis suppurativa and psoriasis”, instead of the term combination.
Also, we provide severity assessment before and after treatment for psoriasis and hidradenitis, for both cases. The clinical improvement is estimated using the PASI (Psoriasis Area Severity Index) score, BSA (Body Surface Area), and IHS4 (International Hidradenitis Suppurativa Severity Scoring System) score, while the impact on the quality of life is estimated with the DLQI (Dermatology Life Quality Index) score.
See the authors' detailed response to the review by Georgios Nikolakis
See the authors' detailed response to the review by Thrasyvoulos Tzellos
See the authors' detailed response to the review by Kevin K. Wu
Hidradenitis suppurativa (HS) and psoriasis are considered chronic inflammatory diseases suggesting the existence of common pathogenetic links1–3. Patients with psoriasis and HS have elevated levels of tumor necrosis factor (TNF) and interleukin-17 (IL-17) in lesional and non lesional tissues, which has been the justification for selective targeting of these inflammatory pathways4–7. We present two cases of co-existence of psoriasis and HS treated with certolizumab pegol and brodalumab due to the peculiarities of treatment with other therapies.
The first patient, a 27-year-old Caucasian woman, presented with extensive psoriasis vulgaris covering her head, trunk, lower limbs over a period of 5 years, with a recent PASI (Psoriasis Area Severity Index) score of 10.5 and 10% BSA (Body Surface Area) score. She, also, suffered from psoriatic arthritis with axial joint involvement (manifestations of hierolagonitis) over the previous 2 years and moderate HS of Hurley II stage disease on the axillae over the last year, with IHS4 (International Hidradenitis Suppurativa Severity Scoring System) score 10 (Figure 1a, b, c, d, e). Despite the limited extent of the lesions, the patient presented considerable pain, discomfort and substantial negative effect on quality of life. Patient’s DLQI (Dermatology Life Quality Index) score was, also very high, 21. The patient didn’t have a positive family history for the above diseases and the molecular control for HLA-B27 was negative. Previous treatments with topical corticosteroids and methotrexate for one year were not effective and treatment with apremilast for 8 months didn’t offer clinical improvement in both diseases. The patient underwent comprehensive laboratory investigations, including complete blood cell count, chemistry panel, tuberculosis (Quantiferon-TB Gold test), human immunodeficiency virus and hepatitis B and C screening and chest x-ray. Since all these examinations revealed values within normal limits and because of the patient’s desire for childbirth, she was treated with certolizumab pegol (CZP). The initial dose was 400mg, followed by 400mg every 2 weeks. Treatment with CZP significantly improved psoriasis and psoriatic arthritis at week 8 and HS at week 12. The PASI score after treatment was 1, BSA was 2%, whereas IHS4 score was 1. Except the clinical improvement, the DLQI score was impressively reduced to 2. (Figure 1f–i). She continues treatment 9 months after and at 3 months follow-up is fully controlled.
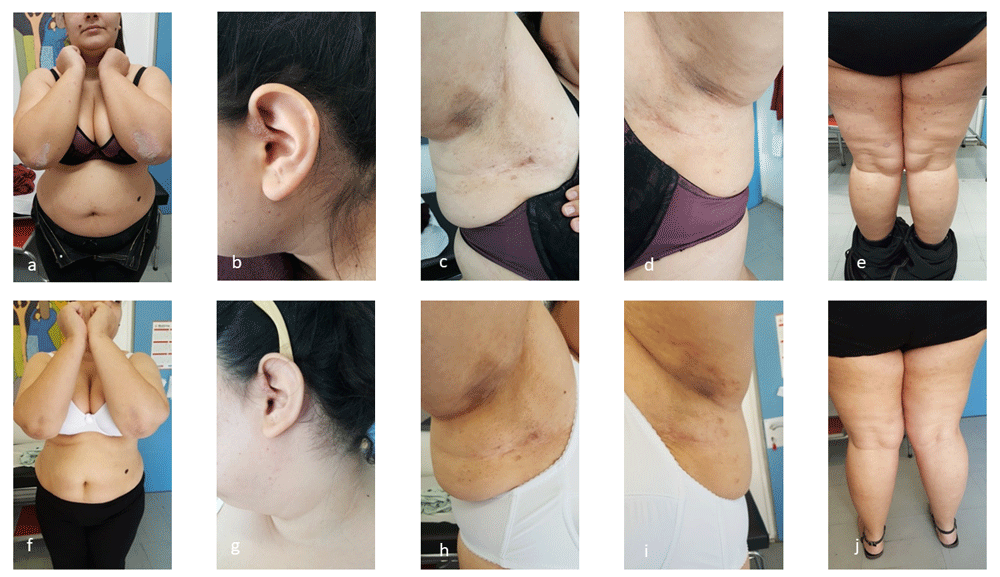
(a–e) Psoriatic and HS lesions of first patient before treatment with certolizumab pegol. (f–j) Psoriatic and HS lesions of first patient after treatment with certolizumab pegol.
The second patient, a 42-year-old Caucasian man, was referred to our hospital’s dermatological department with multiple, itchy, scaly, red-gray psoriatic plaques covering almost all his body: scalp, arms, trunk, thighs (Figure 2a–d) for the previous 6 months, over a history of 10 years psoriatic disease (recent PASI: 18.5, BSA: 45%). The patient, also, experienced concomitant psoriatic arthritis with peripheral joint involvement and dactylitis discomfort over the previous 10 years, and moderate HS of Hurley II stage disease appearing on the groin area in the previous year. The IHS4 score was 10. The above diseases had a negative impact factor on his quality of life with DLQI 25. The patient’s family history was positive: his mother and sister were also suffering from psoriasis. The patient had until recently received almost all the available therapies related to his diseases: cyclosporine for 2 years interrupted due to urea and creatinin increase (examinations restored after discontinuation), methotrexate and golimumab for 3 years with improvement only in psoriatic arthritis, adalimumab ustekinumab and secukinumab, with a partial response. After a complete laboratory examination, with results in normal limits, the patient started therapy with brodalumab. The initial dose was 210 mg at weeks 0, 1, 2 followed by 210 mg every 2 weeks. His psoriasis and psoriatic arthritis were highly improved at week 8 (Figure 2 e–h), as was HS at week 16. The PASI score after treatment was 1.5, the BSA was 8%, while IHS4 score was reduced to 3. He has continued treatment for 1 year; at 3 months follow-up he reported improvement in his quality of life and the DLQI score was 1.
Monoclonal antibody therapies have revolutionized the treatment of chronic inflammatory disorders such as psoriasis and HS. CZP is a TNF inhibitor that does not have a fragment crystallizable (Fc) region, which is normally present in a complete antibody and therefore it does not cause antibody-dependent cell-mediated cytotoxicity8–10. In contrast to other whole-antibody anti-TNFs, CZP crosses the placenta only by passive diffusion and could therefore be considered as the first-line choice of treatment for women who wish to become pregnant. Since CZP is an anti-TNF drug, therapies which have good clinical response in both psoriasis/psoriatic arthritis and HS, it was chosen as the treatment of choice in our case since it also has a safe profile for possible future pregnancy.
Brodalumab is a monoclonal antibody against human IL-17 receptor A (IL-17RA). Given its efficacy in psoriasis and its mechanism of action in psoriatic arthritis and HS, due to the patient’s non response to all the available treatment options it was decided its use on the above combination diseases11–14.
It is well known that psoriasis and HS likely share immunopathogenetic pathways, including involvement of IL-17 and TNF. Given the good clinical response to anti-IL 17 and anti-TNF drugs in psoriasis and HS treatment, investigations into this direction could represent a starting point for a new therapeutic approach for revolutionary treatment of two difficult to treat diseases.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and clinical images was obtained from the patients.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: HS, sebocytes, acne, melanoma, allergy
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Psoriasis, HS, atopic dermatitis, biologics, epidemiology.
Is the background of the cases’ history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the conclusion balanced and justified on the basis of the findings?
No
Competing Interests: Dr Kanni and I both work on collaborating groups on Hidradenitis suppurativa (Athens, Greece and Dessau Germany, respectively). We published together in 2016 in JID (Journal of Investigative Dermatology). This does not affect my current review, it was objective to the best of my knowledge.
Reviewer Expertise: HS, sebocytes, acne, melanoma, allergy
Is the background of the cases’ history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
Competing Interests: Advisory Board and primary investigator for UCB and Abbvie. Do not know about the authors. These conflicts of interest did not influence my review of this manuscript.
Reviewer Expertise: Hidradenitis suppurativa, atopic dermatitis, biologics, Evidence based medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 22 Dec 20 |
read | read | |
|
Version 1 26 Nov 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (1)