Keywords
Tunicate, Oikopleura, Genetic code, Mitochondria, Cytochrome oxidase subunit I
Tunicate, Oikopleura, Genetic code, Mitochondria, Cytochrome oxidase subunit I
Based on Reviewer 2's comments, we expanded our introduction.
See the authors' detailed response to the review by Yuanning Li
Tunicates are marine animals that have acquired the capacity to produce cellulose by horizontal gene transfer approximately 500 million years ago (Matthysse et al., 2004; Nakashima et al., 2004). Together with vertebrates and cephalochordates, they belong to the chordate phylum, in which they share morphological features such as a muscular tail during larval stages. Phylogenetic studies place the tunicates as the closest living relatives of vertebrates (Delsuc et al., 2006). Tunicates can be subdivided in three classes: Thaliacea (free-swimming colonial species, for instance salps or dolioids), Appendicularia (free-swimming solitary species with an adult morphologically similar to the larval stage of other tunicates), and Ascidiacea (attached to solid substrates in their adult stage, for instance sea squirts). The relationship between these classes and therefore their mono- or paraphyly has been revised multiple times. For instance the 18S rRNA analysis of Stach & Turbeville (2002) nested Appendicularia within Ascidiacea, but more recently Delsuc et al. (2018) placed them as sister groups using a multigene approach. The paraphyly of Ascidiacea is now widely accepted, as the above studies and others demonstrated that they contain the Thaliacea.
Mitochondrial genomes undergo major changes at the geological time scale due to their small size and clonal reproduction, including changes to their genetic code (Osawa et al., 1992). In animals, alternative genetic codes have first been found in large clades, for instance echinoderms (Himeno et al., 1987) and hemichordates (Castresana et al., 1998), but more recent works underline the presence of changes deeper in the phylogenetic tree, for instance within nematodes (Jacob et al., 2009) and within hemichordates (Li et al., 2019). The first evidence that ascidians use a specific mitochondrial genetic code stemmed from observations that the cytochrome c oxidase subunit 1 (Cox1) sequence from Halocynthia roretzi (Yokobori et al., 1993) and the Cox3 sequence of Pyura stolonifera (Durrheim et al., 1993) are interrupted by stop codons if translated using the vertebrate mitochondrial code. Reassignment of AGR codons to glycine was later confirmed by the discovery of a glycine (Gly) tRNA in the H. roretzi genome (Yokobori et al., 1999) and by the sequencing of its anticodon (U*CU) (Kondow et al., 1999). Apart from the AGR codons, the ascidian code is similar to the vertebrate and the invertebrate ones, with ATA assigned to methionine (Met) and TGA to tryptophan (Trp) (Yokobori et al., 1993).
This genetic code is known as the “ascidian” genetic code; however, it is also used by non-ascidian tunicates, such as the thaliacean Doliolum nationalis (Yokobori et al., 2005). The possibility that this genetic code emerged earlier than tunicates was raised by the study of partial genome sequences of Branchiostoma lanceolatum (Delarbre et al., 1997) leading to the proposition that AGR might encode Gly in cephalochordates. While this seemed to be supported by the discovery of a putative TCT (Gly) tRNA in the full mitochondrial genome of B. lanceolatum (Spruyt et al., 1998), this hypothesis was later ruled out by an analysis of the related amphioxus Branchiostoma floridae (Boore et al., 1999), and has not been reconsidered since. Finally, studies on the appendicularian branch showed compatibility between the mitochondrial sequence of Oikopleura dioica and the ascidian code (Denoeud et al., 2010). Nevertheless, support for compatibility was not demonstrated explicitly for the ATA and TGA codons and the mitochondrial sequence of O. dioica were not released in International Nucleotide Sequence Database Collaborations (INSDC) databanks.
Cox1 is the most conserved mitochondrial protein. Although no mitochondrial genome has been fully sequenced yet for appendicularians, partial Cox1 sequences are present in the INSDC databanks for Oikopleuridae. Sakaguchi et al. (2017) reported that all Oikopleura mitochondrial sequences (AY116609–AY116611 and KF977307) may be contaminations from bacteria or cnidarians, and provided partial sequences for Oikopleura longicauda in the same study. Partial mitochondrial sequences were published for Bathochordaeus and Mesochordaeus species by Sherlock et al. (2017). In addition, Naville et al. (2019) recently published draft genome for several appendicularian species. Therefore, to assess whether the ascidian mitochondrial code is used across the whole tunicate subphylum, we took advantage of these public data and prepared a curated alignment of Cox1 sequences comprising representatives of the major tunicate branches, to study the consensus sequences at conserved residues.
We identified Cox1 and Cytochrome b (Cob) gene sequences for Oikopleura longicauda, Mesochordaeus erythrocephalus and Bathochordaeus stygius by screening published genome assemblies (Naville et al., 2019) with the partial Cox1 sequence of O. longicauda LC222754.1 (Sakaguchi et al., 2017) using tblastn and the ascidian mitochondrial code (-db_gencode=13) (Gertz et al., 2006). Mitochondrial genome sequences were then translated using the cons and getorf commands from EMBOSS (Rice et al., 2000), using the ascidian mitochondrial code.
We identified the circular contig SCLD01101138.1 (length: 10,324 nt) as a potential mitochondrial genome, and translated Cox1 from position 4530 to 6230. We also translated Cob from 3697 to 4668.
We translated Cox1 in contig SCLF01725989.1 (length 7,034 nt) on reverse strand from position 1792 to 272. Using the same method with O. longicauda’s Cob sequence as a bait, we also recovered a Cob sequence from contig SCLF01109548.1 (length 5,010 nt), reverse strand, 1604 to 2590.
We used the consensus of the published B. stygius Cox1 sequences KX599267.1 to KX599281.1 from GenBank (Sherlock et al., 2017), to screen the genome and scaffold SCLE01415711.1 (length 10,388 nt) gave a perfect hit. We translated Cox1 from position 8054 to 6522 on the reverse strand, and a partial Cob sequence from scaffold SCLE01415711.1 (2319 to 2963, reverse strand). We also found a second fragment aligning well with C-terminal sequences between positions 2373 and 1978, but we did not include it due to the difficulty of resolving the overlap between both fragments. When screening with the M. erythrocephalus Cox1 sequence recovered above, we found that another scaffold SCLE01416475.1 gave a perfect hit, hinting at a possible contamination.
To assemble a Cox1 sequence in O. dioica, we downloaded expressed sequence tags (file 10_ESTall.txt) from Oikobase (Danks et al., 2012) and extracted hits matching the O. longicauda sequence using tblastn (see above). We then aligned and visualised the hits using Clustal Omega (Sievers et al., 2011) and SeaView (Gouy et al., 2009), filtering out those too short or introducing gap columns. Inspection of the alignment let us notice three possible haplotypes. We generated a consensus for each of them, translated them (see above) and trimmed the proteins sequences in order to match the length of the other reference sequences in the alignment. All variants found between the haplotypes were synonymous codons. We used the same methodology to generate a consensus for Cob and translate it.
Bathochordaeus charon KT881544.1 ORF2 translated with ascidian code; Bathochordaeus stygius: SCLE01415711.1[8054:6522] translated with ascidian code; Branchiostoma lanceolatum:BAD93656.1; Caenorhabditis elegans: NP_006961.1; Ciona intestinalis: CAL23359.2; Clavelina oblonga: YP_009029840.1; Doliolum nationalis: BAD86512.1; Halocynthia roretzi: NP_038239.1; Mesochordaeus erythrocephalus: SCLF01725989.1[1915:260] translated with ascidian code; Mus musculus: NP_904330.1; Oikopleura dioica: consensus of Oikobase contigs (see file 10_ESTall.txt) KT0AAA24YA11, KT0AAA22YO17, KT0AAA22YO04, KT0AAA13YK14, KT0AAA18YK22, KT0AAA16YP04, KT0AAA13YE23, KT0AAA8YH10, KT0AAA4YK01, KT0AAA24YE23, KT0AAA18YO18, KT0AAA3YP19, KT0AAA10YF12; O. longicauda: SCLD01101138.1[4678:6230] translated with ascidian code; Salpa thompsoni: BBB04277.1.
Bathochordaeus stygius: SCLE01415711.1[2963:2319] translated with ascidian code; Branchiostoma lanceolatum: BAD93666.1; Caenorhabditis elegans: NP_006958.1; Ciona intestinalis: CAL23352.2; Clavelina oblonga: YP_009029843.1; Doliolum nationalis: BAD86520.1; Halocynthia roretzi: NP_038246.1; Mesochordaeus erythrocephalus: SCLF01109548.1[1604:2590] translated with ascidian code; Mus musculus: NP_904340.1; Oikopleura dioica: consensus of Oikobase contigs KT0AAA23YJ17, KT0AAA16YJ22, KT0AAA17YO14, KT0AAA10YI15, KT0AAA18YI18, KT0AAA11YF07, KT0AAA10YG05, KT0AAA1YH02, KT0AAA12YH10, KT0AAA12YC07, KT0AAA12YC07, KT0AAA18YM15 (see file 10_ESTall.txt); O. longicauda: SCLD01101138.1[3697:4668] translated with ascidian code; Salpa thompsoni: BBB04269.1.
Translated Cox1 and Cob sequences were aligned using Clustal Omega (Sievers et al., 2011) and SeaView (Gouy et al., 2009). The alignments were post-processed using the showalign -show=n command of EMBOSS (Rice et al., 2000) to show the differences to the inferred consensus. Graphical processing of the alignments were performed with Jalview (Waterhouse et al., 2009). The codon sequences encoding Cox1 and Cob of the tunicate species were then added aligned to the corresponding amino-acid (three lines per species, see Extended data (Plessy & Pichon, 2019)) and then the text files were transposed, so that each line would correspond to a single position in the alignment, and interrogated with custom Unix commands to compute the tables presented in this manuscript.
We selected species according to sequence availability and to ensure coverage of the tunicate subphylum in a way that stays broad under the various hypotheses of monophyly or paraphyly for its major groups. For ascidians, we have included the phlebobranchian Ciona intestinalis, the aplousobranchian Clavelina oblonga and the pyurid stolidobranchian Halocynthia roretzi. For thaliaceans, we selected Doliolum nationalis and Salpa thompsoni. For appendicularians we selected Oikopleura dioica, Oikopleura longicauda, Bathochordaeus stygius and Mesochordaeus erythrocephalus. We ensured that all tunicate sequences were translated with the ascidian mitochondrial genetic code. Lastly, we included outgroup sequences from Caenorhabditis elegans and Branchiostoma lanceolatum (invertebrate mitochondrial code) and from Mus musculus (vertebrate mitochondrial code) to better highlight conserved amino acid positions. In Figure 1, we illustrate the relation between these species based on the phylogeny of Naville et al. (2019) for appendicularians and of Delsuc et al. (2018) for the other tunicates. We prepared Cox1 sequences from the selected species using mitochondrial genomes (for ascidians, thaliaceans, and outgroups), from draft genomes in which we found a putative mitochondrial contig after screening with a partial or a related Cox1 sequence (for O. longicauda, B. stygius, and M. erythrocephalus) and from EST sequences (for O. dioica). We aligned the translated Cox1 and Cob sequences (Figure 2 and Figure 3) and inspected the positions where all species use the same amino acid. Conserved glycines supported the use of AGR codons across the whole tunicate clade. We confirmed this observation with Cob sequences obtained with the same method.

Different branch colors indicate different mitochondrial genetic codes. Codon assignments with an equal sign indicate how the nucleotide sequences were translated. Codon assignments with a question mark indicate a possible finding, but were not used for translation. Ascidians, in which the AGR to Gly codon reassignment was initially discovered, are highlighted among the tunicates. Right: codon sequence of Cox1 genes on positions where proposed changes of genetic code would make all species use the same amino acid.
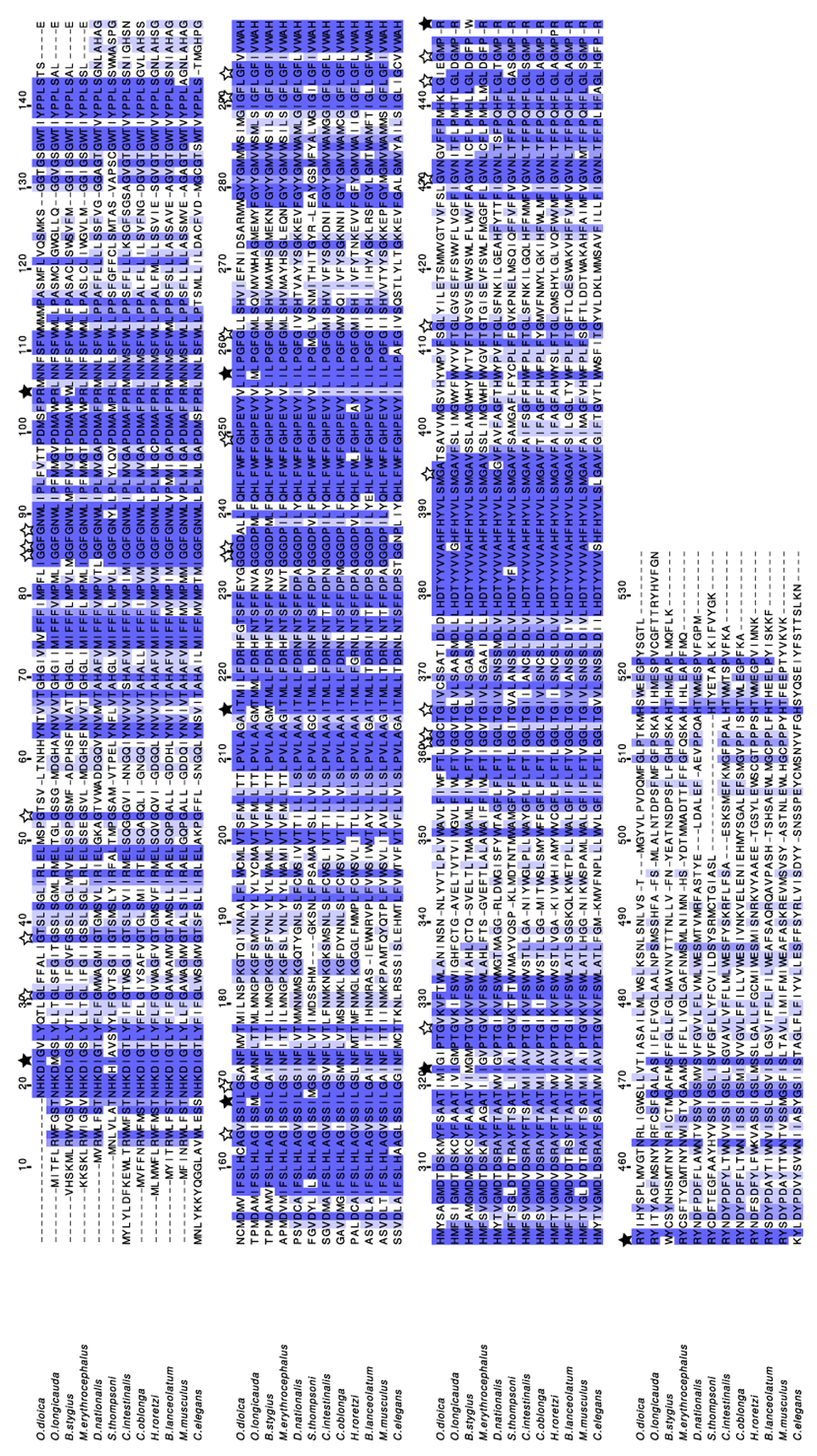
White stars indicate conserved cysteines when at least one tunicate uses an AGR codon. Black stars indicate positions suggesting a different genetic code.
We then searched for positions where a single tunicate species differed from the other sequences with the same replacement amino acid more than once. We found multiple cases of methionine being replaced by isoleucine and arginine replaced by tryptophan in O. longicauda and B. stygius (Figure 2). Given their phylogenetic proximity, we grouped the two species in the analysis below and we calculated the number of mismatches to the other sequences. We redefined a position as “conserved” if there is at most one mismatch from one sequence to the others.
M. erythrocephalus does not seem to use ATA codons and O. longicauda and B. stygius use ATA codons at positions where all other species had an isoleucine (Ile) (Table 1 and Table 2). In the ancestral invertebrate mitochondrial code and the sister vertebrate code, ATA encodes Met. Although Met and Ile both have hydrophobic side chains that often can substitute for each other, this also suggests a change of the genetic code. Evidence for this is that 1) non-appendicularian species do not display ATA codons at positions where all other species encode Ile; 2) the change would be parsimonious as O. longicauda, B. stygius and M. erythrocephalus are more closely related to each other than to O. dioica (Naville et al., 2019); and 3) these three species never have ATA codons at positions where Met is conserved in every species (in contrast to O. dioica). Furthermore, reversion of the ATA codon to Ile have occurred in other branches of the tree of Life, for instance in echinoderms (Jacobs et al., 1988). Finally, inspection of a partial Cox1 sequence of the related Bathochordaeus charon (KT881544.1) provided one extra instance of an ATA codon at a conserved Ile position.
| O. dio. | O. lon. | B. sty. | M. ery. | |
|---|---|---|---|---|
| ATA number | 22 | 12 | 16 | 0 |
| ATC number | 3 | 8 | 0 | 2 |
| ATG number | 14 | 34 | 25 | 33 |
| ATT number | 29 | 18 | 21 | 38 |
| ATA on cons. Met | 5 | 0 | 0 | 0 |
| ATA on cons. Ile | 0 | 4 | 2 | 0 |
| O. dio. | O. lon. | B. sty. | M. ery. | |
|---|---|---|---|---|
| ATA number | 6 | 9 | 9 | 0 |
| ATC number | 5 | 2 | 1 | 2 |
| ATG number | 9 | 9 | 8 | 16 |
| ATT number | 21 | 11 | 11 | 22 |
| ATA on cons. Met | 0 | 0 | 0 | 0 |
| ATA on cons. Ile | 0 | 1 | 1 | 0 |
The TGA codon is known to encode tryptophan (Trp) in vertebrate, invertebrate and ascidian mitochondria (Fox, 1979). We found that B. stygius uses TGA at positions where all other species would encode Arg (Table 3 and Table 4). This is surprising as these two amino acids are unlikely to functionally substitute for each other. O. longicauda does not use TGA codons, and M. erythrocephalus does not use TGA at conserved Arg, although it is found at a position where all other species encode for Arg except C. elegans which encodes lysine, the other positively charged amino-acid. This again suggests a possible change of genetic code, although the numbers are currently too small to draw a solid conclusion.
| O. dio. | O. lon. | B. sty. | M. ery. | |
|---|---|---|---|---|
| TGA number | 2 | 0 | 3 | 1 |
| TGG number | 13 | 16 | 19 | 16 |
| TGA on cons. Trp | 1 | 0 | 0 | 0 |
| TGA on cons. Arg | 0 | 0 | 2 | 0 |
We extracted Cox1 and Cob sequences of four different appendicularians from public databases. As a nucleotide sequence, Cox1 might be useful for mining databases of molecular barcodes sequenced from the environment, or for studies of population diversity within a species. As a protein sequence, Cox1 might be useful for refining the phylogeny of appendicularians. However, a translation code needs to be chosen.
Our alignments of tunicate Cox1 and Cob protein sequences support the view that all tunicates translate AGR codons as Gly (although this conclusion might be limited by the lack of coverage for the Kowalevskiidae and Fritillariidae families). While our analysis suggests that the last common ancestor of the tunicates used the “ascidian” code, it is not possible to conclude that all contemporary tunicates still do, as we found discrepancies on other conserved residues that could be explained by a genetic code change of ATA and TGA codons within a sub-clade of the appendicularians containing M. erythrocephalus, O. longicauda and B. stygius.
The “ascidian” genetic code is table number 13 in the NCBI protein database, where it is used to translate sequences from ascidians and non-ascidian tunicates, for instance D. nationalis. However for appendicularians, the NCBI currently applies the invertebrate table (number 5). This has the consequences of turning Gly to Ser at functionally important positions. Therefore, the ascidian is probably a more appropriate default. At present, it is unclear whether some appendicularians have additional changes; however, the accurate translation of AGR codons to Gly would nonetheless reduce the amount of error in translated protein sequences.
To confirm a change of genetic code, it is necessary to detect corresponding changes in the respective tRNAs. This beyond reach for the present study because the mitochondrial genomic sequences that we used are extracted from draft genome sequences that may be incomplete, or even contain contaminations (see B. stygius in the Methods section). As a result, we also cannot entirely rule out the possibility that we have examined pseudogenes, although the high conservation found in the alignments suggest this in unlikely. For all these reasons, it is necessary to sequence full-length mitochondrial genomes from appendicularians.
Our alignments of translated mitochondrial sequences suggest that the last common ancestor of living tunicates may have already used the “ascidian” genetic code. Thus, we recommend the use of that code instead of the “invertebrate” one for all tunicates in automatic translation pipelines, with the caveat that additional changes might be found in appendicularians. This observation is a reminder that in biology, exception is the rule, and that each time a mitochondrial sequence is extracted from a species for the first time, it is important to carefully examine its genetic code.
All data underlying the results are available as part of the article and no additional source data are required.
Zenodo: Aligned Cox1 and Cob sequences from Oikopleura dioica and other tunicates. https://doi.org/10.5281/zenodo.3490310 (Plessy & Pichon, 2019).
This project contains alignment files and descriptions of how the files were generated.
Extended data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We thank the OIST’s Scientific Computing & Data Analysis Section for their support, and Ferdinand Marlétaz for critical comments on our manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Evolutionary genomics and phylogenetics in marine invertebrates.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tunicate embryology and genomics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 14 Apr 20 |
||
|
Version 1 10 Dec 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)