Keywords
Harm Reduction, Heat-Not-Burn, Modified Risk Tobacco Product, Actual Use, Product Adoption
Harm Reduction, Heat-Not-Burn, Modified Risk Tobacco Product, Actual Use, Product Adoption
Compared to the previous version, the Abstract has been slightly edited to further improve its structure, with no alteration to the message presented. The Introduction was edited to present how other regulatory authorities are considering different types of products, including e-cigarettes, and to provide examples of the MRTPs available in the U.S. (including MRTP applications accepted for review). We also updated in the Introduction the status of the PMI MRTP Applications for IQOS. The version of the product provided for the study is now indicated in the Methods. The Discussion section has been reworked to further reflect on the interpretation of the study results together with limitations when it comes to potential inference. The text of the paper has also been modified to improve the English in some places.
See the authors' detailed response to the review by Riccardo Polosa
See the authors' detailed response to the review by Fabrizio Messina and Krishna Prasad
See the authors' detailed response to the review by Kenneth A. Mundt
AIC: Akaike Information Criterion; CDC: U.S. Centers for Disease Control and Prevention; CRF: case report form; FDA: U.S. Food and Drug Administration; MRTP: modified risk tobacco product; THS: tobacco heating system
Cigarette smoking causes pulmonary, cardiovascular, and other serious diseases and is responsible for the largest number of preventable deaths in the United States (U.S.)1,2. It is widely known that the best way to avoid these risks is to never start smoking. For smokers, the best way to reduce the risks and adverse health consequences of smoking is to quit3. However, as smoking is addictive, smoking cessation has proven difficult to achieve. Despite a decline in the smoking prevalence in the U.S. from 21% to 16% over the last decade, an estimated 40 million people in the U.S. smoked cigarettes in 2015, with around 30% of them smoking menthol cigarettes4.
The U.S. Food and Drug Administration (FDA) and other international health authorities have recognized that in order to more rapidly reduce the burden of death and disease from tobacco use, current tobacco control measures should be enriched and complemented by tobacco harm reduction strategies1,5,6. Tobacco harm reduction strategies aim to provide smokers who do not want to stop nicotine use with alternative, noncombustible tobacco and nicotine-containing products or nicotine delivery systems that eliminate exposure to smoked tobacco and thus substantially reduce harm compared with smoking combustible products7–14.
In the U.S., this has given rise to a regulatory framework for manufacturers to market modified risk tobacco products (MRTP), defined as “any tobacco product that is sold or distributed for use to reduce harm or the risk of tobacco-related disease associated with commercially marketed tobacco products”15.
MRTPs aim to avoid the high level of risks of chronic disease, morbidity, and mortality caused by smoking cigarettes on their users, and their risk profile is an essential factor in estimating the public health effects of these products16. Examples of MRTPs may include tobacco and/or nicotine-containing products such as e-cigarettes, smokeless tobacco, and heated tobacco products. Various authorities (Public Health England, 2018; Royal College of Physicians, 2016; U.S. Department of Health and Human Services, 2014) have concluded that e-cigarettes for example are likely to be substantially safer than cigarettes. Other products (i.e. heated tobacco products) heat tobacco rather than burning it, thus producing far lower quantities of harmful and potentially harmful constituents (HPHC) than are found in cigarette smoke13. While it has been acknowledged that more research on the relative risk of heated tobacco products compared with that of combustible tobacco is needed, the available evidence suggests that heated tobacco products may be considerably less harmful than cigarettes17,18. Currently, the most widely available heated tobacco product is the Tobacco Heating System (THS) developed by Philip Morris International (PMI), sold under the IQOSTM brand name. IQOS was launched in 2014 in Italy and Japan. As of March 31, 2021, IQOS was available in 66 countries19. In July 2020, the FDA authorized the claim ‘AVAILABLE EVIDENCE TO DATE: The IQOS system heats tobacco but does not burn it. This significantly reduces the production of harmful and potentially harmful chemicals. Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals.’20. Other MRTP applications have also been accepted for review, made public, and in some cases authorized by the FDA (i.e. General and Camel snus products, Copenhagen moist snuff product, and a very low nicotine cigarette)21–24.
THS is made up of three distinct components:25 (1) a tobacco stick, specifically designed for use at low temperatures and containing specially processed crimped tobacco, (2) a holder for the THS Tobacco Stick that electronically heats the tobacco and controls the temperature, and (3) a charger for recharging the holder after each use. THS uses a precisely controlled heating system into which the THS Tobacco Stick is inserted to generate an aerosol without combusting tobacco. The device heats tobacco to significantly lower temperatures (no more than 350°C) than cigarettes, thereby significantly reducing or eliminating HPHCs from the inhaled aerosol compared with cigarette smoke. The substantial reduction in toxic emission and subsequent body exposure have been established by the THS manufacturer (PMI) and competitors25–40. Though a few studies have brought contradictory evidence41,42, the weight of evidence produced by independent studies, including FDA laboratory tests, confirms PMI’s findings on the substantial reduction of major carcinogens17,34,43–47. While prevalence data are still sparse, evidence from Japan, where IQOS was first launched, suggests a steady increase in awareness and use of IQOS between 2015 and 201748,49. Analysis of predictors of IQOS current use (use in the previous 30 days) in 2017 showed that current Japanese smokers with intention to quit had higher odds to use IQOS than that of those with no intention to quit (13.3 vs. 6.7), while women aged 60 years or more showed significantly lower odds than reference categories49. Ever-use of e-cigarettes was associated with greater odds of using IQOS. These findings suggest that the large majority of IQOS users in Japan switched from cigarettes to IQOS and that there is minimal uptake from nonsmokers. However, they provide limited information on how IQOS would impact public health in countries other than Japan48,49.
More specifically, in the context of an MRTP application, the FDA recommends assessment of the public health impact of candidate MRTPs under close to real-world conditions to understand how U.S. adult consumers actually use the product50, thus requiring actual use evidence for a product which is not yet commercialized in the U.S. The U.S. Institute of Medicine recommended studies that provide real-world evidence, including ad libitum use of MRTPs alone and in combination with cigarettes7. Although real-world evidence is generally gathered from observational studies in a post-market setting, as with over-the-counter drugs, where consumers are provided with the product together with labeled directions for use51–53, most of the actual use data that have been collected on potential MRTPs have been done in an artificial setting, and the MRTP is provided for free, as opposed to what happens for other commercialized tobacco products in real-life conditions54–56.
The present study reports the findings of a pre-market actual use study performed in the context of IQOS MRTP application to the FDA57. The goal of the study was to measure THS use patterns in U.S. adult daily cigarette smokers and to assess THS product acceptance.
To mimic real-life situations as closely as possible, adult daily smokers had access to THS regular and menthol flavor products and were free to consume cigarettes, THS, and any other nicotine-containing products ad libitum.
The present analysis aims at identifying the potential predictors (i.e., socio-demographics, smoking habits, sensory assessment, and ease of use) of THS adoption in adult cigarette smokers. The effect of THS product flavor (i.e., regular or menthol) was also investigated.
The actual use observational study consisted of one-week baseline period, a six-week observational period, and a one-week close-out period (see Figure 1)57. During the baseline period, participants recorded their regular cigarette consumption. During the subsequent observational period, participants recorded their consumption of both cigarettes and THS. Throughout the entire observational period, all participants were free to consume cigarettes, THS, and any other nicotine-containing products ad libitum. The observational period served to assess the development of THS use patterns. A close-out period was implemented for safety surveillance.
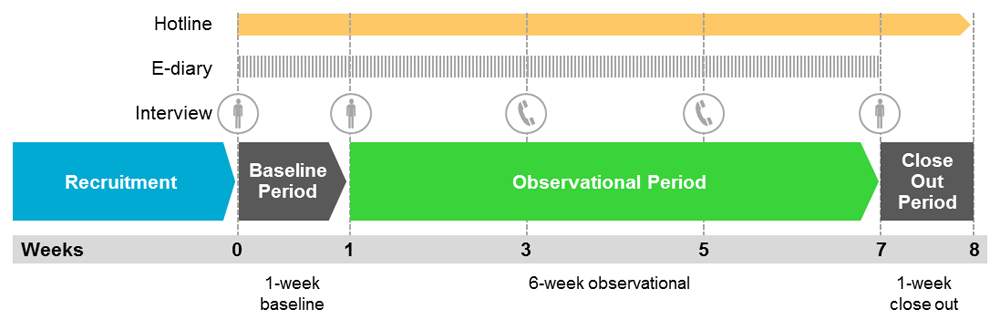
* During the baseline and the observational periods, participants recorded their stick-by-stick consumption of cigarettes and/or THS into an electronic diary (e-diary). Participants were able to call the toll-free telephone hotline to raise queries related to the study, resolve issues related to the e-diary or THS, and report product quality complaints and adverse health events associated with the use of THS.
The study was conducted between 21 September 2015 and 7 January 2016 in eight cities located across the U.S. (Asheville, NC; Charlotte, NC; Denver, CO; Detroit, MI; Las Vegas, NV; Miami, FL; Oklahoma City, OK; Tampa, FL). The study locations were chosen to recruit a sufficiently large and diverse number of current U.S. adult daily smokers. In each city, the research and recruitment agency C&C Market Research operated a dedicated booth within a mall, which was used as a study site. All study materials were reviewed and approved on 28 August 2015 by Sterling Institutional Review Board (ID: 5149-001) before actual study implementation. This study was performed in accordance with Good Epidemiological Practice58.
Study participants were recruited from the C&C Market Research databases. C&C’s databases consist of approximately 400,000 individuals nationwide who are recruited to join the site database via mall intercept, word of mouth, or by visiting the C&C Market Research website. The sampling was designed using quotas in terms of sex (male (56%); female (44%)), age (18–24 years old (34%); 25–44 years old (34%); 45+ years old (32%)), race (white (70%); black or African American (30%)), and income (low (48%); moderate/high (52%))1.
Based on information available for each person (e.g., age, gender, smoker/nonsmoker, etc.) within the database, individuals employed by C&C Market Research randomly contacted potential study participants via telephone. No specific method or particular order was utilized for the selection of study participants beyond ensuring that the quotas were met. Individuals who met the following inclusion criteria were eligible for the study: (a) 18 years of age or above according to the minimum legal age), (b) currently living in the U.S., (c) current daily smokers of regular and/or menthol cigarettes with no intention of quitting within the next 30 days, (d) interest in participating in an eight-week study and providing informed consent. The following individuals were excluded from the study: (a) women who, based on self-report, were either pregnant, breastfeeding, or of childbearing potential and not using adequate means of contraception, and (b) individuals who had started smoking within the last 30 days. Eligible individuals were then invited to a study site, where they were rescreened for eligibility based on their ID document for proof of age and were asked their intention to use THS based on their reading of a multipage information brochure on THS (Extended data59). Only participants with a positive intention (i.e., “somewhat likely”, “very likely”, “definitely” using a six-point Likert scale ranging from “definitely not” to “definitely”) were enrolled in the study.
Sample size calculation was based on a precision-based approach (accuracy in parameter estimation) based on predetermined tightness of the confidence intervals. Given a precision of ± 5% for 95% confidence intervals of prevalence estimates and assuming a proportion of 50% of participants passing a consumption threshold of 100 THS products and 40% attrition, the study aimed to recruit 1,300 participants.
The investigational tobacco product as part of this actual use study was THS Version 2.2 and was provided by PMI. Products available to participants during the observational period had a neutral design with study identification elements to ensure confidentiality of the THS material, given the pre-market nature of the actual use study. U.S. Surgeon General’s warnings were present on each THS pack in a rotating fashion.
At enrollment in the study, participants completed an informed consent form and were interviewed in person by trained staff from the C&C Market Research study site in order to provide information on the purpose and goal of the study and instructions on how to use an electronic diary to report tobacco consumption. Questionnaires were also administered to collect demographic information, such as sex, age, race, ethnicity, education, occupation, and income as well as information on smoking habits, including the average number of cigarettes smoked per day, type of cigarette (menthol, regular), current usage of e-cigarettes, current usage of nicotine replacement therapy products, attempts to quit smoking, and the likelihood as well as the reasons to use THS regularly.
During the one-week baseline period, participants were requested to make an entry into an electronic diary (e-diary) every time they consumed a cigarette. Upon completion of the one-week baseline period, participants returned to the study site to receive THS and choose between THS regular, menthol, or a combination of the two products, according to their taste preference. Participants were provided with a maximum of 100 THS products at the start of the observational period. This supply ensured that all participants had access to THS on the initial days of participation in the observational period. During the remaining study period, participants could request additional THS products. Excessive ordering of additional THS was prevented by fixing an individual maximum number, based on self-reported cigarette consumption assessed at enrollment and then applying an “inflation factor” of three to allow for potential increase of use of THS.
During the six-week observational period, participants were requested to make an entry into the e-diary every time they consumed a THS or a cigarette. If no entries were made until a predefined time point per day, the e-diary sent an acoustic signal and displayed a reminder to record consumption. E-diary data were transferred automatically to a central database each night. In addition, participants were interviewed every two weeks to assess the taste, smell, aftertaste, and ease of use of THS (telephone interviews at Weeks 3 and 5 and personal interview at Week 7). Taste, smell, and aftertaste were assessed using a seven-point Likert scale ranging from one to seven, where one represented “I don’t like it at all”, and seven represented “I like it very much”. Similarly, ease of use was measured using a seven-point Likert scale ranging from one to seven, where one was “not easy to use at all”, and seven was “very easy to use”.
Participants were able to call the toll-free telephone hotline to raise queries related to the study, resolve issues related to the e-diary or THS, and report product quality complaints and adverse health events associated with the use of THS. At the end of the observational period, participants were asked to return all study materials. During the "close-out" week period participants were not required to record any data, however, for the continued surveillance of potential adverse events (AEs), they were able to call the toll-free telephone hotline to report if they experienced any adverse events with the use of THS during the course of the study.
Study participation was voluntary, and participants were free to withdraw at any time. Compensation in the study was based on participants’ level of participation and on compliance with the study procedures (maximum of $440) and paid via check at the end of the study.
The main outcome measure was self-reported consumption of cigarettes and THS during the observational period. This measure was used to derive a variable describing the percentage of THS use on a weekly basis by dividing the number of THS products by the number of total tobacco products used (THS products plus cigarettes). In order to facilitate meaningful description and interpretation of THS use patterns and future comparison across various studies60, this product use variable was then trichotomized into the following predefined usage categories: (1) THS use (≥ 70% of total tobacco product used being THS[70–100]% THS), (2) combined use (> 30% to < 70% of total tobacco product used being THS ]30–70[% THS), and (3) cigarette use (≤ 30% of total tobacco product used being THS [0–30]% THS). In addition, “Adoption of THS” at Week 6 was defined as ≥ 70% of THS products in a participant’s combined consumption of tobacco products during Week 6.
The following variables were evaluated as potential predictors of THS adoption (Table 1):
| Total1 | Adoption of THS | No adoption of THS | p-value for Chi-square | ||
|---|---|---|---|---|---|
| All participants | 965 (100%) | 141 (14.6%) | 824 (85.4%) | . | |
| Demographics2 | |||||
| Sex | Male | 474 (49.1%) | 81 (17.1%) | 393 (82.9%) | 0.0323 |
| Female | 491 (50.9%) | 60 (12.2%) | 431 (87.8%) | ||
| Age in categories | 18 to 24 years | 223 (23.1%) | 24 (10.8%) | 199 (89.2%) | 0.1671 |
| 25 to 44 years | 363 (37.6%) | 59 (16.3%) | 304 (83.7%) | ||
| Above 44 years | 379 (39.3%) | 58 (15.3%) | 321 (84.7%) | ||
| Persons in household in categories | 1 person | 216 (22.4%) | 38 (17.6%) | 178 (82.4%) | 0.1591 |
| > 1 person | 749 (77.6%) | 103 (13.8%) | 646 (86.2%) | ||
| Children in household in categories | None | 615 (63.9%) | 96 (15.6%) | 519 (84.4%) | 0.2586 |
| 1 or more children | 348 (36.1%) | 45 (12.9%) | 303 (87.1%) | ||
| Marital status | No relationship | 729 (75.5%) | 114 (15.6%) | 615 (84.4%) | 0.1126 |
| Relationship | 236 (24.5%) | 27 (11.4%) | 209 (88.6%) | ||
| Occupational status | At work | 597 (61.9%) | 87 (14.6%) | 510 (85.4%) | 0.9520 |
| Not at work | 367 (38.1%) | 54 (14.7%) | 313 (85.3%) | ||
| Educational attainment | Low and moderate | 452 (46.9%) | 72 (15.9%) | 380 (84.1%) | 0.2822 |
| High | 512 (53.1%) | 69 (13.5%) | 443 (86.5%) | ||
| Income levels | Low | 334 (36.1%) | 54 (16.2%) | 280 (83.8%) | 0.2424 |
| Moderate | 413 (44.7%) | 62 (15.0%) | 351 (85.0%) | ||
| High | 177 (19.2%) | 19 (10.7%) | 158 (89.3%) | ||
| Socio-economic status | Low and moderate | 339 (36.7%) | 54 (15.9%) | 285 (84.1%) | 0.3934 |
| High | 584 (63.3%) | 81 (13.9%) | 503 (86.1%) | ||
| Race | White | 653 (67.8%) | 88 (13.5%) | 565 (86.5%) | 0.1376 |
| Black or African American / Other | 310 (32.2%) | 53 (17.1%) | 257 (82.9%) | ||
| Ethnicity | Hispanic or Latino | 115 (11.9%) | 27 (23.5%) | 88 (76.5%) | 0.0041 |
| Not Hispanic or Latino | 850 (88.1%) | 114 (13.4%) | 736 (86.6%) | ||
| Study location | Asheville | 119 (12.3%) | 11 (9.2%) | 108 (90.8%) | 0.1194 |
| Charlotte | 109 (11.3%) | 10 (9.2%) | 99 (90.8%) | ||
| Denver | 134 (13.9%) | 21 (15.7%) | 113 (84.3%) | ||
| Detroit | 121 (12.5%) | 14 (11.6%) | 107 (88.4%) | ||
| Las Vegas | 121 (12.5%) | 22 (18.2%) | 99 (81.8%) | ||
| Miami | 124 (12.8%) | 25 (20.2%) | 99 (79.8%) | ||
| Oklahoma City | 111 (11.5%) | 16 (14.4%) | 95 (85.6%) | ||
| Tampa | 126 (13.1%) | 22 (17.5%) | 104 (82.5%) | ||
| Smoking behavior | |||||
| Average number of cigarettes per day in categories | 1–10 cigarettes | 405 (42.0%) | 72 (17.8%) | 333 (82.2%) | 0.0318 |
| 11–20 cigarettes | 439 (45.5%) | 58 (13.2%) | 381 (86.8%) | ||
| ≥ 21 cigarettes | 121 (12.5%) | 11 (9.1%) | 110 (90.9%) | ||
| Usage of e-cigarettes | No | 913 (94.6%) | 129 (14.1%) | 784 (85.9%) | 0.0756 |
| Yes | 52 (5.4%) | 12 (23.1%) | 40 (76.9%) | ||
| Intention to quit smoking within the next 6 months | No and don't know | 929 (96.3%) | 134 (14.4%) | 795 (85.6%) | 0.4027 |
| Yes | 36 (3.7%) | 7 (19.4%) | 29 (80.6%) | ||
| Last attempt to quit smoking | Some time in the past | 391 (40.5%) | 43 (11.0%) | 348 (89.0%) | 0.0087 |
| Never | 574 (59.5%) | 98 (17.1%) | 476 (82.9%) | ||
| THS Tobacco Sticks type ordered | Only regular THS Tobacco Sticks | 365 (37.8%) | 43 (11.8%) | 322 (88.2%) | 0.0069 |
| Only menthol THS Tobacco Sticks | 424 (43.9%) | 59 (13.9%) | 365 (86.1%) | ||
| Both THS Tobacco Sticks types | 172 (17.8%) | 39 (22.7%) | 133 (77.3%) | ||
| THS Tobacco Sticks consumption type not available | 4 (0.4%) | 0 | 4 (100%) | ||
| Product assessment | |||||
| Sensory assessments (taste, smell, aftertaste)3 | First quartile (< 2.0) | 225 (24.0%) | 13 (5.8%) | 212 (94.2%) | < .0001 |
| Second quartile (2.0 to < 3.5) | 288 (30.7%) | 27 (9.4%) | 261 (90.6%) | ||
| Third quartile (3.5 to < 5.0) | 209 (22.3%) | 35 (16.7%) | 174 (83.3%) | ||
| Fourth quartile (≥ 5.0) | 215 (22.9%) | 63 (29.3%) | 152 (70.7%) | ||
| Ease of use4 assessment | Not easy to use (1,2,3) | 301 (32.1%) | 18 (6.0%) | 283 (94.0%) | < .0001 |
| Quite easy to use (4,5) | 276 (29.5%) | 33 (12.0%) | 243 (88.0%) | ||
| Easy to use (6,7) | 360 (38.4%) | 87 (24.2%) | 273 (75.8%) |
1 n = 965, excluding three participants without any reported Tobacco Stick or cigarette use within Week 6. Only nonmissing data are shown in the table.
2 Categories recorded in the case report form (CRF) were condensed in order to reduce the number of estimators and balance the number of subjects per category: Persons in household in categories: 1 person, > 1 person
Children in household in categories: None; 1 or more children. Information on children in household was missing for two participants.
Marital status: Relationship (CRF categories: Living with someone / Married), No relationship (CRF categories: Never married / Legally separated / Divorced / Widowed)
Occupational status: At work (CRF category: working now), Not at work (CRF categories: Only temporarily laid off, sick leave or maternity leave / Looking for work, unemployed / Retired /Disabled, permanently or temporarily / Homemaker, keep housing / Student / Other). Information on occupational status was missing for one participant.
Educational attainment: Low (CRF category: less than high school diploma) / moderate (CRF category: high school diploma), High (CRF categories: some university training or university degree). Information on educational attainment was missing for one participant.
Income levels: Low (CRF categories: Less than $30,000), moderate (CRF categories: $30,000 to less than $60,000), High (CRF categories: $60,000 and more). Information on income level was missing for 41 participants.
Socio-economic status is derived as a combination of income levels and educational attainment: Low (low income and low education), Moderate (low income and moderate education, low income and high education, moderate income and low education, and high income and low education), and High (moderate income and moderate education, moderate income and high education, high income and moderate education, and high income and high education). Information on socio-economic status was missing for 42 participants.
Race: White, Black or African American/Other (CRF categories: American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander). Information on race was missing for two participants.
Last attempt to quit smoking: Some time in the past (CRF categories: less than 6 months ago, more than 6 months ago), Never.
3 The taste, smell, and aftertaste of the product were assessed using a seven-point scale ranging from 1 = “I don’t like it at all” to 7 = “I like it very much”. For the scale assessments, Cronbach’s alpha was calculated as measure of internal consistency among the scales. Because of an alpha of 0.89 (above the threshold value of 0.8), a combined construct of sensory acceptance was calculated using the mean scale assessments over taste, smell, and aftertaste. Four categories were created based on the quartiles of the distribution of these mean scale assessments. Information on sensory assessment was missing for 28 participants.
Demographics. From the demographic collection at enrollment, the following variables were derived: sex, age (18–24 years, 25–44 years, above 44 years), race (white, black or African American/Other), ethnicity (Hispanic or Latino, not Hispanic or Latino), income (low, moderate, high), number of persons (1 person, > 1 person) and children (none, 1 or more children) in household, marital status (no relationship, relationship), occupational status (at work, not at work), educational attainment (low/moderate, high), socio-economic status (low/moderate, high). In addition, study site location (eight cities) was also considered as a potential demographic predictor.
Smoking behavior. From the smoking habits questionnaire at enrollment, the following variables were derived: average number of cigarettes per day (1–10 cigarettes, 11–20 cigarettes, ≥ 21 cigarettes), usage of e-cigarettes (yes, no), intention to quit smoking within the next six months (no or don’t know, yes), last attempt to quit smoking (some time in the past, never). In addition, the type of THS products ordered through the study observational period was also considered as a predictor of THS adoption (only regular, only menthol, both types).
Product assessment. Taste, smell, and aftertaste assessment collected at the end of the study (Week 7) were aggregated to quantify sensory assessment into four quartiles. Ease of use assessment was aggregated into three categories (not easy to use, quite easy to use, easy to use).
The study population for analysis included all participants who (1) fulfilled all eligibility criteria, (2) had at least one documented consumption of a cigarette during the baseline period, and (3) had at least one documented consumption of a THS product during the observational period.
Potential predictors of THS adoption underwent bivariate screening using the Chi-squared test (see Table 1). Predictors with a p-value < 0.2 were subsequently subjected to stepwise logistic regression, with sex, age, and THS product types ordered being forced-in variables. Backward selection was applied to identify the final model, with p < 0.05 as the selection threshold to retain variables. The resulting model was compared with the model identified by forward selection using the same variables. In case of a difference between the models, the better model based on the Akaike Information Criterion (AIC) was chosen61.
Additionally, the process was repeated using two-way interaction terms between THS product types ordered and each independent variable with p-value < 0.2 from the bivariate screening with simple logistic regressions. The two resulting multiple logistic regression models with and without interaction terms were compared using the AIC.
Analysis was conducted using SAS, version 9.4 (SAS Institute Inc. Cary, NC, USA). All analyses were descriptive and exploratory. No imputation of missing data was applied. Percentages were calculated as proportion of each category based on all non-missing values.
Out of the database managed by C&C Market Research, 8,858 members were contacted via telephone. Of these, 1,860 refused to continue the telephone conversation, 5,630 did not meet the eligibility criteria, and the remaining 1,368 were invited to the closest study site and rescreened against inclusion/exclusion criteria to verify eligibility. Of the 1,336 participants who were enrolled into the study, 1,106 participants self-reported at least one cigarette during the baseline period and at least one THS product during the observational period. At the end of the observational period (Week 6), 968 participants had reported data in e-diaries. Of these, three participants reported use of zero THS products or cigarettes. Thus, the analysis population consisted of 965 participants.
The proportion of male participants (49%) in the analysis population was very similar to the proportion of female participants (51%). More than 75% of the participants were 25+ years old, about two thirds (68%) were white, and slightly more than half (56%) had a yearly household income below $45,000 (Table 1).
Of the analysis population (965 participants), 424 participants (43.9%) ordered only menthol THS products, 365 participants (37.8%) ordered only regular THS products, and 172 participants (17.8%) ordered both types.
The proportion of participants with THS use decreased between Week 1 (19.4%) and Week 6 (14.6%). Usage patterns of THS products were relatively stable in Weeks 4, 5, and 6 of the observational period.
The proportion of participants with combined use (> 30% and < 70% THS) decreased from 41.5% at Week 1 to 22.4% at Week 6, while the proportion of participants with cigarette use (≤ 30% THS) increased from 39.0% at Week 1 to 62.7% at Week 6.
The number of tobacco products (THS products and cigarettes) consumed per day during the observational period was lower than the number of cigarettes consumed per day during the baseline period across all participant groups at Week 6. The mean (± standard deviation) number of tobacco products decreased from 9.0 ± 5.89 to 8.1 ± 5.37 in participants with THS use, from 9.3 ± 6.34 to 8.9 ± 6.21 in participants with combined use, and from 10.9 ± 7.69 to 9.9 ± 6.75 in participants with cigarette use (Table 2).
At the end of the observational period (Week 6), 14.6% of the analysis population had adopted THS use (Table 1). The proportion of participants adopting THS was higher in males (17.1% vs. 12.2%), in participants aged more than 25 years (25 to 44 years: 16.3%, above 44 years: 15.3% vs. 18 to 24 years: 10.8%), in one person households (17.6% vs. 13.8%), in participants with no relationship (15.6% vs. 11.4%), in black or African Americans (17.1% vs. 13.5%), and in Hispanic or Latino participants (23.5% vs. 13.4%).
With regard to smoking habits, the proportion of participants adopting THS was higher in participants smoking from one to 10 cigarettes per day (17.8% vs. 11 to 20 cigarettes per day: 13.2% and ≥ 21 cigarettes per day: 9.1%), e-cigarette users (23.1% vs. 14.1%), and in participants who never attempted to quit smoking (17.1% vs. 11.0%). The proportion of participants who adopted THS was higher in those who ordered both THS products (22.7% vs. 13.9% for menthol only vs. 11.8% for regular only).
The proportion of participants adopting THS was higher in participants who liked the taste, smell, and aftertaste of THS (increasing from 5.8% in the first quartile to 29.3% in the fourth quartile for sensory assessment scores) and in participants who found THS easy to use (increasing from 6.0% in participant who found THS not easy to use to 24.2% in participants who found THS easy to use).
Stepwise main effects logistic regression analysis resulted in the same model, max-rescaled R-square of 0.1968 and 76.2% of concordant pairs, regardless of the selection method (i.e., forward or backward). The predictors of adoption of THS at the end of the observational period are summarized in Figure 2.
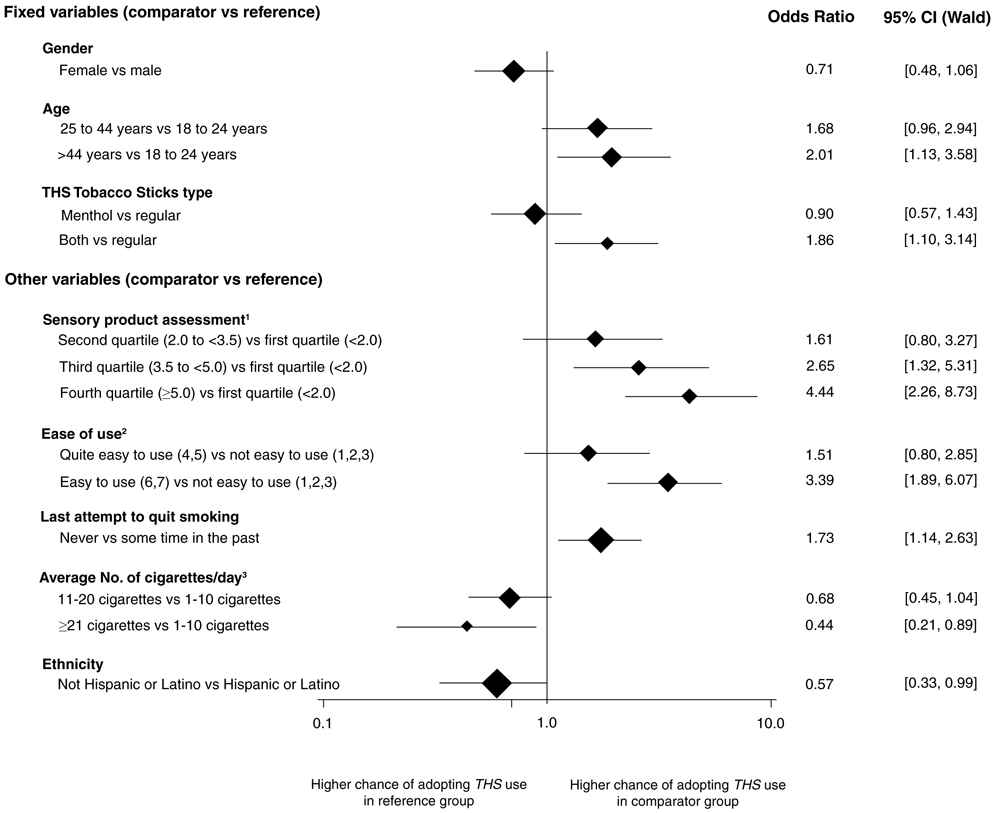
The vertical line shows the value where chances of adopting are equal in both the reference and the comparator group. Horizontal lines show the confidence intervals. The size of the diamonds is proportional to the number of participants in the comparator group. 1 The taste, smell, and aftertaste of the product were assessed using seven-point scales ranging from 1 = “I don’t like it at all” to 7 = “I like it very much”. For the scale assessments, Cronbach’s alpha was calculated as measure of internal consistency among the scales. Because of an alpha of 0.89 (above the threshold value of 0.8), a combined construct of sensory acceptance was calculated using the mean scale assessments over taste, smell, and aftertaste. Four categories were created based on the quartiles of the distribution of these mean scale assessments. 2Ease of use of the product was assessed using a seven-point scale ranging from 1 = “not easy to use at all” to 7 = “very easy to use”. 3Average number of cigarettes/day at enrollment.
No influence of sex (OR = 0.71 [95% CI: 0.48–1.06]) was found, but adoption of THS was more likely in participants aged more than 44 years (OR = 2.01 [95% CI: 1.13–3.58]) and in participants who ordered both THS product types (OR = 1.86 [95% CI: 1.10–3.14]).
Sensory assessment and ease of use were the main predictors for THS adoption. The odds of adopting THS were more than four times higher in participants who liked the smell, taste, and aftertaste of THS (≥ 5.0 points on a seven-point scale) (OR = 4.44 [95% CI: 2.26–8.73]). Similarly, the odds to adopt THS were more than three times higher in participants who found THS easy to use (OR = 3.39 [95% CI 1.89–6.07]).
Participants who had never attempted to quit smoking had a higher chance of adopting THS compared with those who attempted to quit at some time in the past (OR = 1.73 [95% CI 1.14–2.63]).
Participants who smoked on average ≥ 21 cigarettes/day had a lower chance of adopting THS compared with those who smoked on average 1–10 cigarettes/day (OR = 0.44 [95% CI 0.21–0.89]), and the same applied for non-Hispanic or Latino participants compared with Hispanic or Latino participants (OR = 0.57 [95% CI 0.33–0.99]) (Figure 2). Interaction terms with the consumed THS product type did not improve the overall model fit.
The main goal of this study was to describe THS adoption in a real-world setting and to identify potential predictors for adoption of THS. This actual use study was conducted in U.S. adult daily smokers and included 1,106 participants self-reporting their consumption of cigarettes and/or THS products using an e-diary.
During the observational period, the proportion of participants with THS use was stable from Week 4 onwards and by Week 6, almost 15% of the participants had adopted THS, suggesting that THS is a viable alternative to cigarettes for adult smokers. The results do not indicate an increase of overall tobacco consumption over the observational period. Therefore, even though dual use is likely to happen in the first weeks of THS use, it is unlikely to lead to higher abuse liability and increase exposure to tobacco and nicotine products.
The adoption of THS was higher in participants ordering both THS types compared with participants ordering only regular or only menthol THS, suggesting that the availability of several variants of THS, including menthol, might result in a higher proportion of U.S. adult smokers substituting cigarettes with THS. Similar findings have been reported in studies with e-cigarettes and noncombustible nicotine products62–65. Some of these studies also indicated that the use of menthol can facilitate the transition from cigarettes to MRTPs, such as heated tobacco products62–65
Participants who liked the smell, taste, and aftertaste of THS and participants who found THS easy to use were more likely to adopt THS, compared with participants who did not like THS smell, taste, and aftertaste or did not find THS easy to use. This finding supports results from previous studies that found that one of the main reasons that people stop using e-cigarettes after trying them is that they do not like the taste65–68.
Participants smoking 1–10 cigarettes per day were more likely to adopt THS than participants smoking more than 21 cigarettes/day. A similar outcome has been reported for e-cigarettes, as indicated by the prevalence of regular use of e-cigarettes being higher among adult smokers who smoke a lower number of cigarettes per day69.
Participants who never attempted to quit smoking in the past were more likely to adopt THS than participants who had previously attempted to quit smoking. Intention to quit smoking within the next six months was not associated with THS adoption suggesting that the availability of THS is unlikely to interfere with intention to quit smoking.
The proportion of THS adoption was higher in participants aged 44 years and older compared with participants aged between 18 and 24 years old. Hispanic or Latino participants had a slightly higher likelihood of adopting THS than non-Hispanic or Latino participants.
Other demographic characteristics, such as sex, household size, educational attainment, income levels, or race, were not associated with THS adoption.
Overall, these findings show that the socio-demographic characteristics of smokers who are more likely to adopt THS tend to differ from what has been recently reported on e-cigarettes, particularly in terms of age, ethnicity, and previous quit attempts69. This suggests that THS may be seen as an acceptable substitute for cigarettes to a different category of smokers than those who are currently using e-cigarettes. This is corroborated by the fact that current e‑cigarette use was not associated with THS adoption.
Importantly, the study findings highlight the importance of offering alternatives that are close to cigarettes from a sensory experience for the adoption of MRTPs, such as heated tobacco products65–68, with product liking and ease of use being more important predictors for adoption of THS than socio-demographic characteristics and smoking habits.
The key strengths of this actual use study included (1) the high ecological validity due to the near to real-world setting of the study, (2) the broad regional coverage, (3) the large sample size, and (4) the duration of the observational period of six weeks (which is slightly longer than in previous studies of alternative tobacco products70,71).
Limitations include the fact that due to the study having been conducted in a pre-market setting, the study participants did not pay for the THS products, while they continued to pay for their cigarettes, which may have overestimated the level of THS adoption in this study. While important insights were generated regarding the group of current adult daily smokers who participated in the study, the sample was not representative of the U.S. adult smoker population, which should be considered when interpreting the results. Finally, no biochemical verification of tobacco consumption, such as CO monitoring, was used, as the method of data collection relied exclusively on self-reported tobacco consumption. With regard to this point, it should be noted that validation studies have shown that self-reported tobacco consumption behaviors among adults are consistent and reliable55,72.
Factors that were not measured may have influenced THS adoption (e.g., repeated exposure to product communication, peer-to-peer information sharing, risk perception [the product possibly being perceived as possible risk-reduced], familiarity, and acceptability of alternative tobacco usage behavior, as it may develop once the product has been marketed for some time)73.
In view of the above limitations, post-market studies are needed to provide actual levels and drivers of THS adoption and use patterns once THS is commercially marketed in the U.S. Consistent with several theoretical frameworks that have been used to understand the impact of intervention or prevention policies74,75, research should not only look at factors intrinsic to the users or to the product to explain use behavior but also take into consideration the influence of social (e.g., family background, peer influence) and societal/environmental factors (e.g., media influence, public health policy).
This actual use study showed that after a six week period of ad libitum use of THS provided at no expense, almost 15% of U.S. daily adult smokers in the sample replaced 70% or more of their tobacco consumption with THS. The main predictors of THS adoption were positive sensory assessment and the ease of use. Socio-demographic characteristics and smoking habits appeared much less important. The findings suggest that the introduction of THS in the U.S. has the potential to result in adoption by adult smokers who would otherwise continue to smoke cigarettes. On the basis of this adoption rate, this could benefit public health by having a positive impact on this particular population of adult smokers76. In particular, the results indicate that the adoption of THS is unlikely to result in an increase of tobacco consumption. Epidemiologic and post-marketing studies can provide further insights on the levels and the drivers of THS adoption and the use patterns of the THS to allow to assess the impact of the THS at the individual and the overall population level.
Open Science Framework: Potential predictors of adoption of the Tobacco Heating System (THS) by U.S. adult smokers. https://doi.org/10.17605/OSF.IO/SBDXG59.
This project contains the following underlying data files:
Open Science Framework: Potential predictors of adoption of the Tobacco Heating System (THS) by U.S. adult smokers. https://doi.org/10.17605/OSF.IO/SBDXG76.
This project contains the following extended data files:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors would like to thank Kantar Health for assistance in data analysis and medical writing support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Heinze G, Dunkler D: Five myths about variable selection.Transpl Int. 2017; 30 (1): 6-10 PubMed Abstract | Publisher Full TextCompeting Interests: I am a full time employee of British American Tobacco and coordinator for the CORESTA (Cooperation Centre for Scientific Research Relative to Tobacco) product use behaviour (PUB) sub-group.
Reviewer Expertise: I have several years’ experience in tobacco and nicotine products use behaviour. As part of my current role I look after all human studies from mouth level exposure, puffing topography, consumer risk perception and post market surveillance.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: RP has received lecture fees and research funds from Pfizer and GlaxoSmithKline, manufacturers of stop smoking medications. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., and Health Diplomats. RP is the Director of the Center of Excellence for the acceleration of Harm Reduction at the University of Catania (CoEHAR), which has received a grant from Foundation for a Smoke Free World to support 8 research projects. RP is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League)and Chair of the European Technical Committee for standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4).
Reviewer Expertise: Tobacco research (including ECIG and THP)
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: I have no competing interests. Intermittently (ending in early 2018), I provided Philip Morris (sponsor of this study) epidemiological consulting support on study design and interpretation, unrelated to the current study and manuscript.
Reviewer Expertise: Epidemiology with a primary focus on concepts and methods as used to evaluate occupational, environmental and consumer product exposures.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 14 Jul 21 |
|||
|
Version 1 24 Feb 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)