Keywords
chronic kidney disease, end-stage renal disease, quality of life, healthcare costs, disease severity, systematic review, humanistic burden, economic burden
chronic kidney disease, end-stage renal disease, quality of life, healthcare costs, disease severity, systematic review, humanistic burden, economic burden
Chronic kidney disease (CKD) is characterized by a gradual loss of kidney function over time. With an estimated global prevalence of 11–13%, CKD is a common condition that is associated with a significant economic burden across the world1. The prevalence of the disease is rising, owing in part to an increase in the median age of populations worldwide, and the growing number of individuals with diabetes mellitus (DM) or hypertension1. These conditions are the two main causes of CKD and are commonly present in patients with diminished renal function2. In the USA, for example, approximately 40% of people with CKD also have DM, and 32% of people with CKD have hypertension3.
When CKD progresses, patients may experience complications such as anaemia, cardiovascular disease (CVD), peripheral arterial disease, pruritus, and increased risk of infection. Both disease progression and its associated complications require medical treatment, which further impacts patients’ quality of life (QoL) and contributes to the humanistic and economic burden of CKD4,5. Moreover, progression to end-stage renal disease (ESRD) has a significant effect on patients’ daily lives and is often associated with considerable costs due to the common requirement for renal replacement therapy (RRT) via dialysis or kidney transplantation1. Therefore, slowing the rate of progression of CKD to advanced stages, in particular to ESRD, is an important medical objective4.
Many studies have investigated the humanistic and economic burden of CKD, although quantification of this remains challenging owing to differences between methodologies and patient populations across studies. To gain a better understanding of the current evidence, and evidence gaps, we conducted systematic reviews (SRs) to identify relevant evidence on the humanistic burden of CKD, defined here as the effect of CKD on patients’ QoL, and the economic burden, comprising resource use and healthcare costs associated with CKD. In particular, we reviewed data on QoL and costs for patients with CKD compared with the general population, and across disease stages.
Two systematic searches, one on humanistic burden/QoL and one on economic burden, were performed in MEDLINE and MEDLINE In-Process, Embase, and the Cochrane Library via Ovid. Cut-off dates were January 2002 and May 2017 for the humanistic burden SR and January 2007 and May 2017 for the economic burden SR; the shorter review window for the economic burden SR was specified because economic data are considered to decrease in relevance more quickly than QoL data. As supplementary searches, congress abstracts published between 2015 and 2017 (or the most recent 2 years available) from relevant health economics and outcomes research and nephrology meetings were reviewed. The search strings used to identify evidence are listed in Extended data: Supplementary content 16.
Abstracts and titles identified were screened by an independent reviewer to determine whether they met the PICOS (patient, interventions, comparisons, outcomes, and study design) eligibility criteria (Table 1), in accordance with 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines7. All publications that met entry criteria for review were obtained as full articles and reassessed against the predefined inclusion criteria. Owing to the large number of citations meeting these criteria, a decision was taken to restrict the extraction of data from eligible studies. For both SRs, data were extracted only from full publications. This meant that, although congress abstract screening was performed, no data from congress abstracts were extracted. For the humanistic burden SR, study publication dates for data extraction were restricted to 2007–2017; for the economic burden SR, the relevant time period was restricted to 2012–2017. Furthermore, for the humanistic burden SR, data were not extracted if the study population included fewer than 100 patients with CKD or if the study did not use any of the following instruments:
36-item Short-Form Health Survey (SF-36)
12-item Short-Form Health Survey (SF-12)
8-item Short-Form Health Survey (SF-8)
6-dimension Short-Form Health Survey (SF-6D)
5-dimension EuroQol questionnaire (EQ-5D)
Healthy Days/Health-Related Quality of Life (HRQoL) questionnaire
Kidney Disease Quality of Life Questionnaire (KDQoL).
For each publication, information was extracted into a data extraction table. Studies were listed according to inclusion of QoL, symptom burden or cost, and resource use data. For each study, patients’ CKD stage, RRT status [pre-dialysis, dialysis (including dialysis modality) or renal transplant], age, and comorbidities including CVD and the presence of DM were recorded. No risk of bias assessment between or within studies was performed.
These SRs were conducted to collect information on the overall impact of CKD development and progression on patients’ QoL or their healthcare costs. Therefore, we chose to focus this review on studies that compared QoL or costs for patients with CKD and individuals without CKD, or that compared by CKD severity, defined by either disease stage or estimated glomerular filtration rate (eGFR) category. Other studies identified in the SRs are grouped into key themes and listed separately to indicate the scope of the evidence identified in our searches.
In total, 5219 papers were identified in the initial searches, of which 1114 papers were removed as duplicates, and 4105 were included for screening by abstract and title. This screening identified 3539 papers that did not meet the inclusion criteria. In total, 444 papers were included for full paper review. Following full paper review, 284 references were identified for inclusion, plus 79 abstracts identified in supplementary searches. In total, data were extracted from 95 references reporting QoL data, 47 references reporting cost and resource use data, and eight references reporting descriptions of symptoms. A PRISMA flow diagram is shown in Figure 1. Details of the patient populations in the studies discussed in this review are given in Table 2. Figure 2 shows all studies identified in these SRs, grouped into key themes according to the data reported.
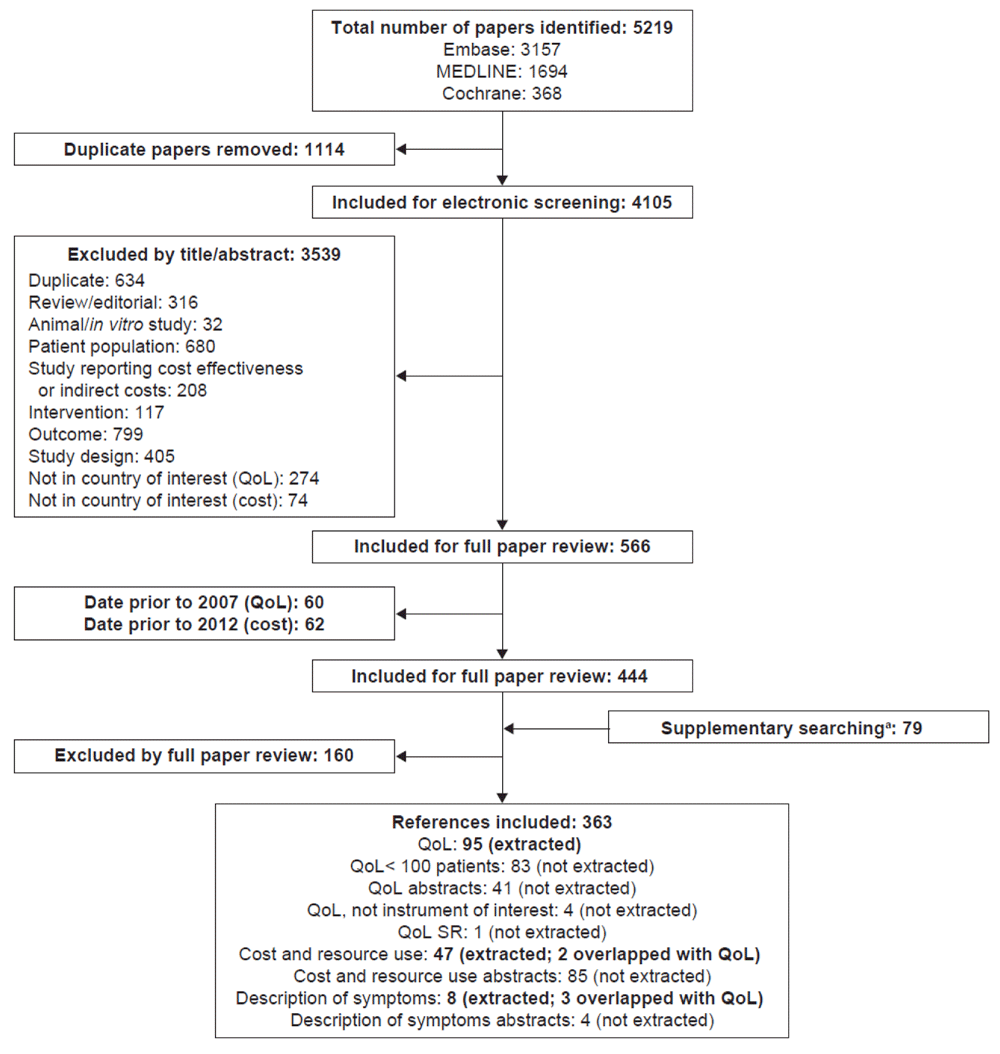
aThe congresses included in this search were the International Society for Pharmacoeconomics and Outcomes Research US and European congresses, the European Renal Association–European Dialysis and Transplant Association congress, the World Congress of Nephrology, the American Society of Nephrology Kidney Week, and the National Kidney Foundation Spring Clinical Meeting. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. QoL, quality of life. SR, systematic review.
| Study characteristics | Lowest severity (or reference category) | Highest severity | Data | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Quality of life in patients with CKD compared with the general population | |||||||||
| Agarwal 2011 USA | Instrument: KDQoL Dialysis: NR DM: CKD: 59% ESRD: 40% | No CKD | vs | Patients with CKD or ESRD | KDQoL sleep quality | ||||
| Boini 2011 France | Instrument: KDQoL Dialysis: NR DM: NR | General population (France) General population (USA) | vs | ESRD | SF-12 PCS | ||||
| SF-12 MCS | |||||||||
| Davison 2009 Canada | Instrument: SF-6D Dialysis: 34% DM: 43% | General population (community- dwelling subjects) | vs | CKD stage 4– 5 | SF-6D | ||||
| Nguyen 2017 USA | Instrument: HRQoL-4 Dialysis: NR DM: 32% | No CKD | vs | CKD stage 3a–5 | HRQoL-4 | ||||
| Wan 2015 China | Instrument: SF-12 Dialysis: 100% DM: NR | General population (Hong Kong) | vs | ESRD | SF-12 PCS | ||||
| SF-12 MCS | |||||||||
| All SF-12 domains | |||||||||
| Wang 2016 China | Instrument: SF-36 Dialysis: 100% DM: NR | National average | vs | ESRD | SF-36 PCS | ||||
| SF-36 MCS | |||||||||
| All SF-36 domains | |||||||||
| Yong 2009 China | Instrument: SF-36 Dialysis: dialysis group: 100% palliative care group: none DM: 42% | General population (Hong Kong) | vs | ESRD (dialysis) ESRD (palliative care) | All SF-36 domains | ||||
| Quality of life in patients with increasing severity of CKD | |||||||||
| Campbell 2013 USA | Instrument: SF-8 Dialysis: excluded DM: 100% (type NR) | eGFR ≥ 90 mL/ min/1.73 m2 | 75–89 | 60–74 | 45–59 | 30–44 | eGFR ≤ 29 mL/min/1.73m2 | SF-8 PCS | |
| SF-8 MCS | |||||||||
| Eriksson 2016 (A) France, Germany, Italy, Spain, UK | Instrument: EQ-5D, SF-12 Dialysis: 35% DM: 100% (type 2) | CKD stage 3 | CKD stage 4 | Dialysis | EQ-5D | ||||
| SF-12 PCS | |||||||||
| SF-12 MCS | |||||||||
| McClellan 2010 USA | Instrument: SF-12 Dialysis: NR DM: 21% (type NR) | eGFR > 90 mL/ min/1.73 m2 | 60–89 | 45–59 | 30–44 | eGFR 15–29 mL/min/1.73m2 | SF-12 PCS | ||
| SF-12 MCS | |||||||||
| Porter 2012 USA | Instrument: SF-36 Dialysis: NR DM: 9% (type NR) | eGFR > 60 mL/ min/1.73 m2 | 50–60 | 40–50 | 30–40 | eGFR < 30 mL/min/1.73m2 | SF-36 PCS | ||
| SF-36 MCS | |||||||||
| Rajan 2013 USA | Instrument: SF-36/SF-12 Dialysis: 3%a DM: 100% (type NR) | CKD stage 0/1 | 2 | 3a | 3b | 4 | 5 | ESRD/dialysis | SF-36/12 PCS |
| SF-36/12 MCS | |||||||||
| Roumelioti 2010 USA | Instrument: SF-36 Dialysis: CKD group: 0% ESRD group: 100% DM: 35% | CKD | vs | ESRD | SF-36 PCS | ||||
| SF-36 MCS | |||||||||
| SF-36 domains | |||||||||
| Wolfgram 2017 USA | Instrument: EQ-5D Dialysis: NR DM: excluded | eGFR ≥ 60 mL/ min/1.73 m2 | > 44–< 60 | eGFR ≤ 44 mL/min/1.73 m2 | EQ-5D | ||||
| Costs and resource use in patients with CKD compared with the general population | |||||||||
| Kim 2016 USA | Dialysis: pre-dialysis or dialysis DM: normal kidney function: 19% pre-dialysis CKD: 33% ESRD: 54% Other: hip fracture | Normal kidney function | CKD, pre-dialysis | ESRD (dialysis or renal transplant) | Hospitalizations; LoS; hospital cost | ||||
| Kumar 2012 USA | Dialysis: pre-dialysis or dialysis DM: normal kidney function: 18% pre-dialysis CKD: 34% ESRD: 38% Other: pulmonary embolism | Normal kidney function | CKD, pre-dialysis | ESRD (dialysis) | Hospitalizations; LoS | ||||
| Puenpatom 2017 USA | Dialysis: NR DM: no CKD: 23% CKD/ESRD: 62% Other: hepatitis C | No CKD | vs | CKD or ESRD | Total healthcare costs | ||||
| Qian 2017 USA | Dialysis: 0% DM: no CKD: 25% CKD: 29% Other: bone metastases | No CKD | vs | CKD stage 3–5 | Total and constituent healthcare costs; hospitalizations; ED visits; outpatient visits; LoS | ||||
| Wyld 2015 Australia | Dialysis: pre-dialysis DM: no CKD: 7% CKD stage 1–2: 31% CKD stage 3–5: 15% | No CKD | vs | CKD stage 1–2, 3, or 4–5 | Direct healthcare costs | ||||
| Costs and resource use in patients with increasing severity of CKD | |||||||||
| Wyld 2015 Australia | Dialysis: pre-dialysis DM: no CKD: 7% CKD stage 1–2: 31% CKD stage 3–5: 15% | No CKD | CKD stage 1–2 | CKD stage 3 | CKD stage 4–5 | Direct healthcare costs | |||
| Turchetti 2016 Italy | Dialysis: pre-dialysis DM: 35% | CKD stage 4 | vs | CKD stage 5 | Direct medical costs | ||||
| Smith 2016 USA | Dialysis: NR DM: 37% | CKD stage 3a with comorbidities | CKD stage 3b | CKD stage 4–5 | Total insurance claims costs | ||||
| McQueen 2017 USA | Dialysis: included DM: 100% (type NR) | CKD stage 1 | CKD stage 2 | CKD stage 3a | CKD stage 3b | CKD stage 4b | CKD stage 5c | Annual costs | |
| Vupputuri 2014 USA | Dialysis: pre-dialysis at baseline DM: 100% (T2) | CKD stage 0–2, no disease progression | CKD stage 0–2, any disease progression | CKD stage 3, no disease progression | CKD stage 3, any disease progression | CKD stage 4, no disease progression | CKD stage 4 with progression to ESRD (dialysis/renal transplant) | Total medical costs (baseline and incremental follow-up) | |
| Ariyaratne 2013 Australia | Dialysis: included DM: CKD stage 1–2: 21% CKD stage 3: 31% CKD stage 4–5: 53% Other: percutaneous coronary intervention | CKD stage 1–2 | CKD stage 3 | CKD stage 4–5 | Total direct cardiovascular costs | ||||
| Eriksson 2016 (B) Sweden | Dialysis: pre-dialysis, dialysis, and renal transplant DM: > 30% except the transplanted group | CKD stage 4–5, pre-dialysis | vs | ESRD (HD, PD, or renal transplant) | Total and constituent costs; hospitalizations; outpatient visits; LoS | ||||
| Escuredo-Vilaplana 2013 Spain | Dialysis: pre-dialysis and PD DM: NR Other: anaemia | CKD stage 2–5, pre-dialysis | vs | PD | Monthly cost of ESAs | ||||
CKD, chronic kidney disease. DM, diabetes mellitus. eGFR, estimated glomerular filtration rate. EQ-5D, 5-dimension EuroQol questionnaire. ESA, erythropoietin-stimulating agent. ESRD, end-stage renal disease. HD, haemodialysis. HRQoL-4, 4-item Healthy Days/Health-Related Quality of Life questionnaire. KDQoL, Kidney Disease Quality of Life questionnaire. LoS, length of stay. MCS, mental component summary. NR, not reported. PCS, physical component summary. PD, peritoneal dialysis. SF-6D, 6-dimension Short-Form Health Survey. SF-8/-12/-36, 8-/12-/36-item Short-Form Health Survey. aTaken as those reported as in the ESRD/dialysis category. b14.4% of patients at CKD stage 4 in this study were receiving dialysis. c70.3% of patients at CKD stage 5 in this study were receiving dialysis.
Several studies compared the QoL of patients with CKD with that of individuals in the general population. These studies reported overall measures associated with QoL, as well as physical component summary (PCS), mental component summary (MCS), and individual domain scores from the SF-36.
Across the studies identified, patients with CKD experienced lower physical and mental QoL compared with the general population. A study by Davison et al. that measured QoL using the SF-6D found that a mixed population of patients with CKD who were either pre-dialysis (CKD stage 3–4, anticipated to require dialysis within 12 months; 34%) or dialysis-dependent (66%) reported lower QoL than an age-matched subset of the general population (SF-6D score: 0.67 vs 0.79)8. A study by Nguyen et al. found that patients with pre-dialysis CKD (stages 3–5), the majority (69.1%) of whom had CKD stage 3a, spent, on average, a greater number of days over a 30-day period inactive owing to poor physical and mental health (3.1 vs 1.5 days; P < 0.001) and a greater number of days with poor physical health (5.1 vs 3.0 days; P < 0.01) compared with a sample of the US general population9. However, a study conducted by Agarwal et al. in the USA, which measured only sleep quality, found no difference between patients with pre-dialysis CKD or dialysis-dependent ESRD and a matched population without CKD10.
Compared with the general population, patients receiving dialysis experienced lower QoL. In total, four studies reported SF-12/SF-36 scores in patients receiving dialysis compared with the general population11–14. Studies by Wan et al. and Wang et al. reported a statistically significant reduction in SF-12 and SF-36 PCS scores (9.9% and 37.6%, reductions, respectively; both P < 0.001; Figure 3)12,13. Boini et al. also reported a reduction in SF-12 PCS scores in patients undergoing dialysis compared with the general population of the USA and France (21.0% and 21.5%, respectively; Figure 3); however, no statistical analysis was reported11. Boini et al.11 and Wang et al.13 reported a decrease in SF-12 (USA: 14.2%; France: 9.1%; P = NR) and SF-36 (23.0%; P < 0.001) MCS scores, respectively, in patients who were dialysis-dependent compared with the general population, whereas Wan et al.12 reported a statistically significant increase in SF-12 MCS scores (9.8%; P < 0.001) in patients receiving dialysis compared with the general population of Hong Kong (Figure 3).
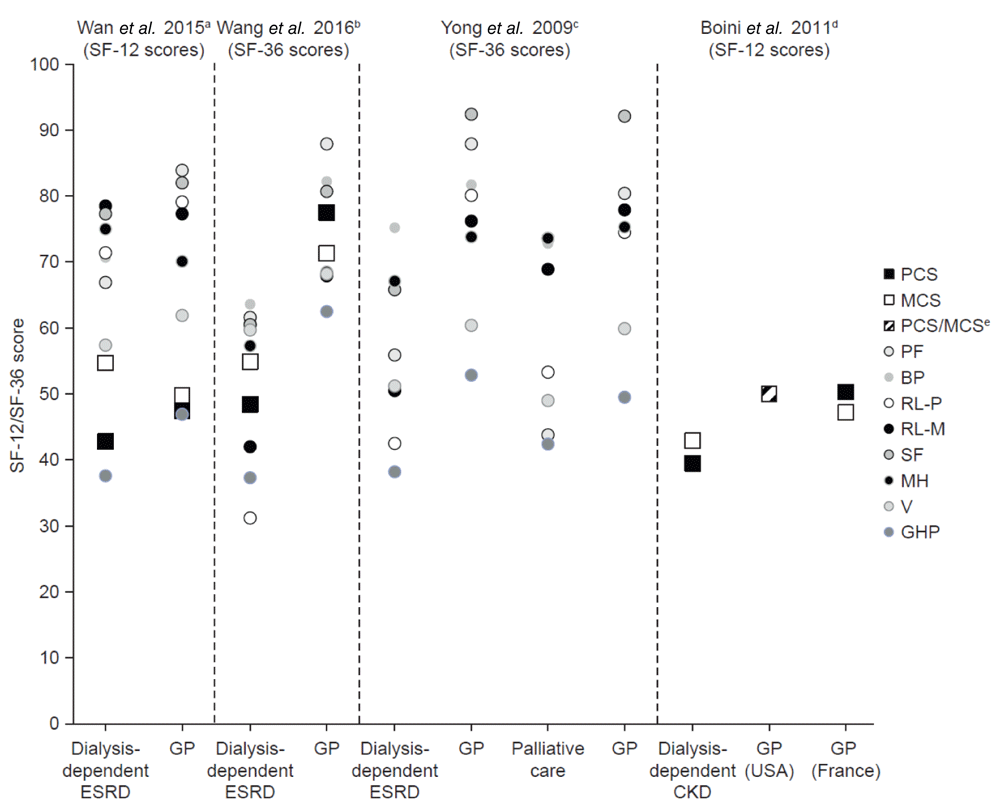
aMH, P < 0.01 versus GP; RL-P and BP, P < 0.005 versus GP; PCS, MCS, PF, and GHP, P < 0.001 versus GP. bFor all domains, P < 0.001 versus average. cPatients with ESRD versus GP (age- and sex-adjusted): BP, P < 0.05 versus GP; MH, P < 0.005 versus GP; PF, RL-P, GHP, V, SF, RL-M, P < 0.001 versus GP. Patients on palliative care versus GP (age- and sex-adjusted): RL-P and V, P < 0.05 versus GP; PF and SF, P < 0.001 versus GP. dNo statistical analysis reported. eFor the general population of the USA, MCS and PCS scores were both reported as 50. BP, bodily pain, CKD, chronic kidney disease. ESRD, end-stage renal disease. GP, general population. HD, haemodialysis. MH, mental health. PF, physical functioning. RL-M, role-limiting (mental). RL-P, role-limiting (physical). SF, social functioning. SF-12/36, 12-/36-item Short-Form Health Survey. V, vitality.
The studies by Wan et al., Wang et al., and Yong et al. reported individual SF-12 and SF-36 domain scores for patients undergoing dialysis compared with the general population12–14. Wan et al. found that patients receiving dialysis reported significantly higher scores for the mental health domain (7.0%; P = 0.006) but lower scores in the physical functioning (20.3%; P < 0.001), role-limiting (physical; 9.8%; P = 0.004), bodily pain (10.0%; P = 0.004), and general health (20.0%; P < 0.001) domains than those without CKD (Figure 3)12. In studies by Wang et al. and Yong et al., a statistically significant reduction in scores across all domains of the SF-36 (P < 0.001 and P < 0.05, respectively) was reported in patients receiving dialysis compared with the general population (Figure 3)13,14. Yong et al. also reported lower SF-36 domain scores in patients with ESRD undergoing palliative care compared with the general population. A statistically significant difference was found only in the physical functioning (P < 0.001), role-limiting (physical; P = 0.027), social functioning (P < 0.001), and vitality (P = 0.036) domains (Figure 3)14.
Several studies reporting QoL data stratified by CKD stage were identified, in addition to studies that examined the impact of demographic factors and comorbidities on the relationship between disease severity and QoL.
Overall, later stages of CKD were associated with worse QoL, but the trends for physical QoL were slightly different from those for mental QoL. In a study by Roumelioti et al., patients receiving maintenance dialysis had a near-significant decrease (17.7%; P = 0.05) in the SF-36 physical functioning domain score compared with patients with advanced CKD (eGFR < 30 mL/min/1.73 m2)15. Similarly, in two US studies by McClellan et al. and Porter et al., decreasing eGFR was associated with lower SF-12 (P = 0.001) and SF-36 (P = 0.004) PCS scores, but there was no apparent relationship between eGFR and MCS scores (data shown in Extended data: Supplementary content 216–18. McClellan et al. reported results from an unadjusted analysis as well as from an analysis adjusted for sociodemographic status and comorbidities; a correlation between eGFR and SF-12 PCS score was identified in both analyses (P < 0.001)16. Porter et al. reported results from an unadjusted analysis only17.
In several studies, the presence of comorbidities affected whether worsening CKD severity was linked to reductions in QoL. Porter et al. found that patients with a greater number of comorbidities experienced overall worse physical and mental QoL, assessed by SF-36 scores, than those with fewer comorbidities (mean PCS/MCS score: no comorbidities, 43.0/47.9; one comorbidity, 39.3/45.8; two or three comorbidities, 36.8/44.4; four or more comorbidities, 31.2/36.0; P < 0.001 for PCS and MCS)17. Furthermore, studies conducted by Campbell et al. and Wolfgram et al. in the USA found that adjustment for comorbidities and sociodemographic factors affected the relationship between QoL scores and eGFR19,20. Campbell et al. found that in an unadjusted analysis, patients with an eGFR of less than 60 mL/min/1.73 m2 reported a statistically significant reduction in SF-8 PCS scores (P < 0.05) compared with patients with an eGFR greater than 90 mL/min/1.72 m2 (reference group), whereas those with an eGFR of 60–89 mL/min/1.72 m2 reported higher SF-8 PCS scores (P < 0.05) than the reference group [mean PCS score by eGFR (mL/min/1.72 m2): ≥ 90, 44.04; 75–89, 44.97; 60–74, 45.00; 45–59, 42.32; 30–44, 40.41; ≤ 29, 40.02]19. However, following full adjustment for demographic factors and comorbidities, there were no significant differences between patients in any eGFR category and the reference group (Extended data: Supplementary content 2)18. Interestingly, there was a statistically significant difference in SF-8 MCS scores between patients who had the most severe disease (eGFR less than 29 mL/min/1.73 m2) and patients with the least severe disease in both unadjusted and adjusted analyses (fully adjusted analysis data shown in (Extended data: Supplementary content 2)18,19. Similarly, a study by Wolfgram et al., also in the USA, found that decreasing eGFR was associated with significantly lower EQ-5D scores in both the unadjusted analysis [mean EQ-5D score by eGFR (mL/min/1.72 m2): ≥ 60, 0.85; > 44 to < 60, 0.85; ≥ 44, 0.82] and an analysis adjusted for sociodemographic factors [mean EQ-5D score by eGFR (mL/min/1.72 m2): ≥ 60, 0.84; > 44 to < 60, 0.84; ≥ 44, 0.82]20. However, in an analysis adjusted for sociodemographics and comorbidities, including CVD, no relationship between eGFR and QoL was found [mean EQ-5D score by eGFR (mL/min/1.72 m2): ≥ 60, 0.73; > 44 to < 60, 0.75; ≥ 44, 0.73]20.
Studies by Rajan et al. and Eriksson et al. assessed the impact of increasing disease severity in patient populations with specific comorbidities21,22. Rajan et al. reported QoL scores for patients with DM that was either prevalent (longer than 3 years) or recent-onset (less than 3 years)22. In both patient populations, scores for the physical functioning and role-limiting (physical) domains of the SF-36 were lower with increasing CKD stage; however, scores for the mental health domain increased up to stage 3, but were lower for stage 4 and lower still for stage 5 (Extended data: Supplementary content 2)18,22. The authors acknowledged that many of the patients with CKD stage 0/1 had claims for International Classification of Diseases (Ninth Revision) codes relating to mental illness, which may account for the lower scores in the mental health domain in this subgroup22. Eriksson et al. reported QoL in patients with pre-dialysis CKD (stage 3–4) or receiving dialysis, with or without anaemia21. Lower SF-12 and EQ-5D scores were reported by patients with CKD stage 4 than those with CKD stage 3, and the lowest scores were reported by patients receiving dialysis; however, no statistical analysis of the difference between stages was reported. This trend was present both in patients with anaemia and in those without anaemia (Figure 4)21.
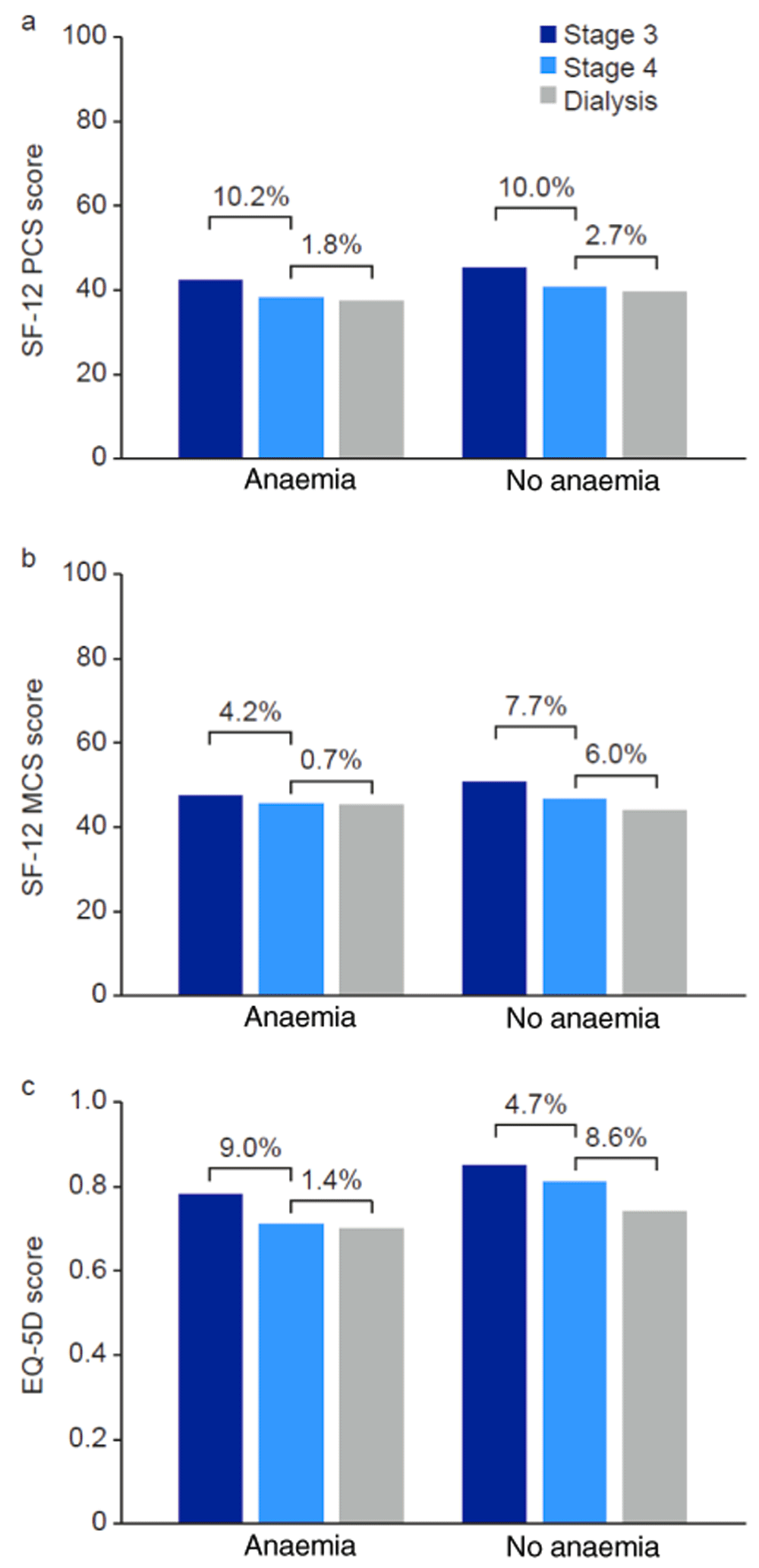
No statistical analysis of the between-stage difference was reported. EQ-5D, 5-dimension EuroQol questionnaire. MCS, mental component summary. PCS, physical component summary. SF-12, 12-item Short-Form Health Survey.
Studies were identified that reported QoL data associated with treatment modality and setting8,12,23–41; comorbidities in CKD17,20–22,41–60; mental health61,62; sleep quality10,59,63–65; patient support, care, and disease management11,66–83; sociodemographic factors84,85; and other factors predictive of CKD development15,86–103. These studies are listed in Extended data: Supplementary content 3104. A total of eight studies were identified on symptom burden in patients with CKD, of which two studies105,106 discussed the symptoms experienced by patients with CKD stage 5 who were not receiving dialysis, and six studies24,34,67,107–109 discussed symptom burden in patients receiving dialysis (Extended data: Supplementary content 3104).
The SR identified several studies that compared costs and resource use between individuals with CKD and those with normal kidney function. Some of the studies differentiated between patients with pre-dialysis CKD and those who required RRT, and the patients in several of the studies had additional comorbidities.
Across all studies, pre-dialysis CKD and ESRD requiring RRT were associated with significant increases in cost and resource use. Kim et al. found that patients with pre-dialysis CKD were significantly more likely to experience hip fracture-related hospitalization than patients with normal kidney function, and those receiving RRT were at higher risk than patients with pre-dialysis CKD. Median hospital costs were significantly greater in patients with CKD than for the general population and were highest for patients requiring RRT. This trend was also observed in the length of hospital stay, although the absolute differences between groups were small (Figure 5)110. Kumar et al. found very similar results in a study examining hospitalization rates and length of stay for pulmonary embolism in patient populations with normal kidney function, with pre-dialysis CKD or receiving dialysis (Figure 5)112. In a study of patients with hepatitis C, total healthcare costs were 2.9 times higher for those who had CKD, compared with those who had hepatitis C without CKD (USD 548 vs USD 1922; P < 0.001)112. Some patients with CKD in this study were defined as having ESRD; however, patients’ dialysis or transplant status was not specified. In a fourth study, patients with bone metastases and impaired kidney function incurred 60% higher total healthcare costs than a control group of patients with bone metastases and normal kidney function (USD 142,267 vs USD 88,839; P < 0.001). These increased costs were driven by hospital admission, emergency department and outpatient visits, longer length of stay in hospital, and higher pharmacy costs113. An Australian study by Wyld et al. also showed that patients with any stage of pre-dialysis CKD incurred significantly higher costs than individuals with no CKD (Figure 6a)114.
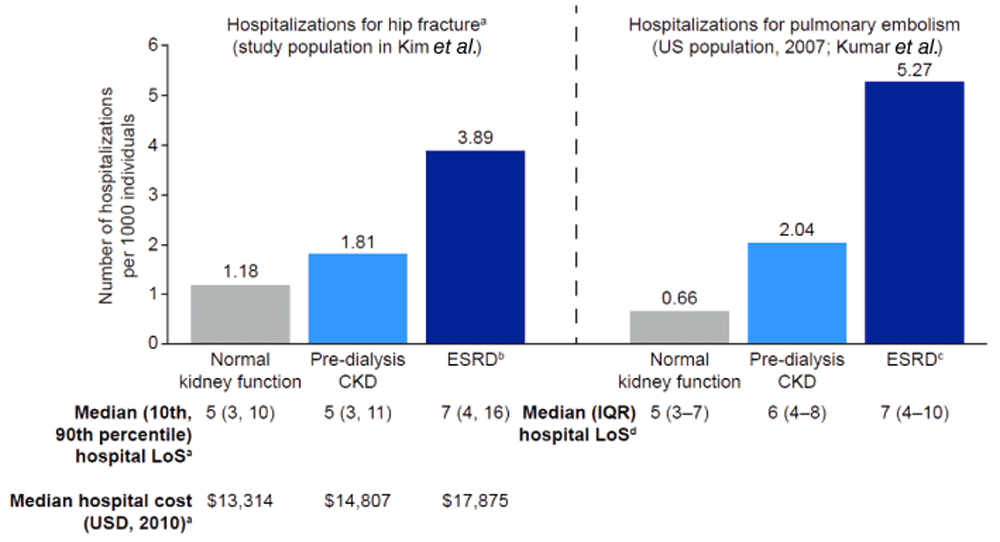
aP < 0.001 for each pairwise comparison. bIncluded patients receiving dialysis or renal transplant. cIncluded patients receiving dialysis. dP < 0.05 versus normal kidney function. CKD, chronic kidney disease. ESRD, end-stage renal disease. IQR, interquartile range. LoS, length of stay. USD, US dollars.
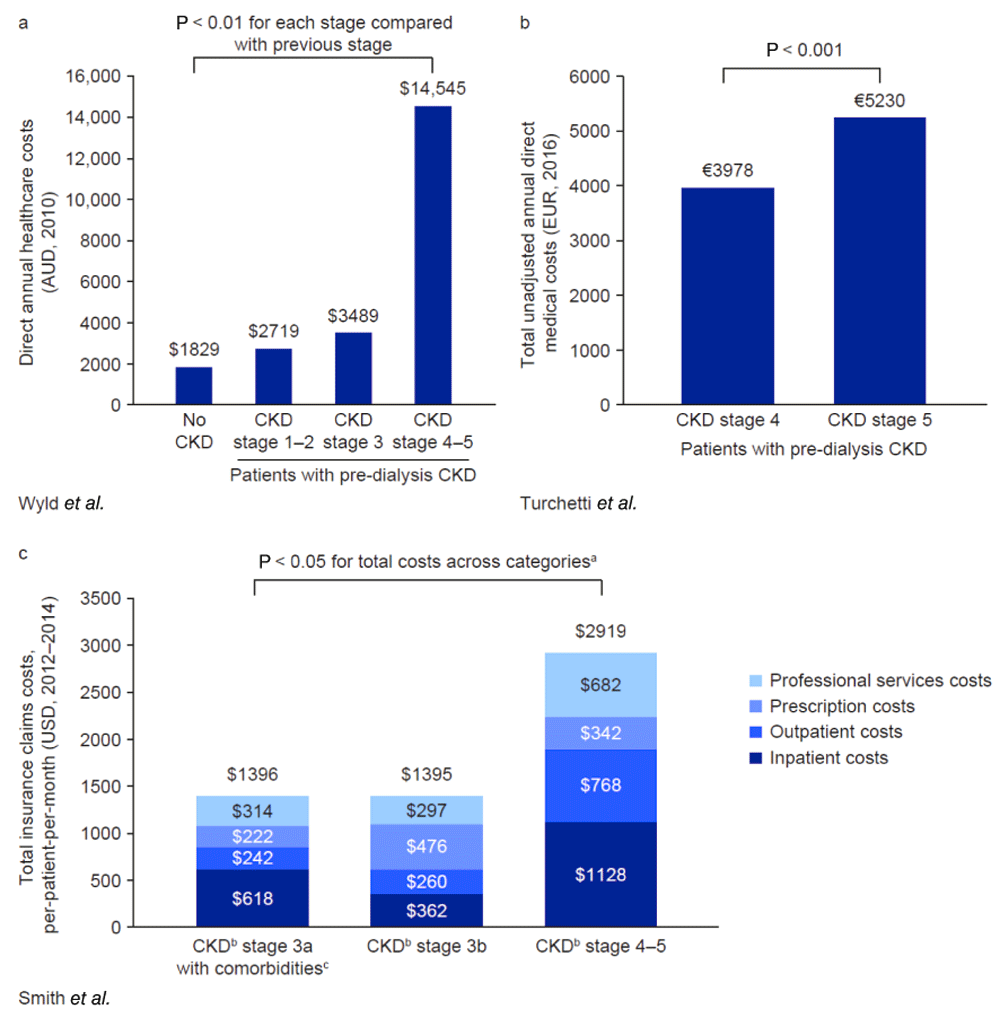
aOne-way ANOVA. bDialysis status not specified. cHypertension, DM, history of CHF, hyperkalaemia, or proteinuria. ANOVA, analysis of variance. AUD, Australian dollars. CHF, congestive heart failure. CKD, chronic kidney disease. DM, diabetes mellitus. EUR, euros. USD, US dollars.
In total, six studies reported costs for patient populations stratified by CKD stage. Across the evidence base, later-stage disease was associated with comparatively higher costs, both in studies including only patients with pre-dialysis CKD and in those in which some patients were receiving RRT.
Wyld et al. showed that costs increased significantly across pre-dialysis CKD stages (Figure 6a)114. Patients with CKD stage 3 incurred approximately 28% higher direct annual healthcare costs than those with CKD stage 1–2; however, there was a much larger cost increase for CKD stage 4–5, with patients in this group incurring more than fourfold higher costs than patients with CKD stage 3. This cost increase was apparent in subgroups of patients both with and without DM, suggesting that the high costs associated with CKD stage 4 and 5 were not attributable solely to the presence of DM. However, only 18 patients in this study had CKD stage 4–5, meaning that the results may have been skewed by a small number of individuals who incurred exceptionally high healthcare expenditure.
Two studies examined the costs of CKD across stages 4–5 or 3–5. An Italian study by Turchetti et al. of patients with pre-dialysis CKD showed a 31% increase in the direct annual medical costs associated with CKD stage 5, compared with CKD stage 4 (P < 0.01; Figure 6b)115. DM and CVD were shown to have an impact on costs incurred by patients at either CKD stage. Smith et al., who conducted a study in the USA, found that patients with CKD stage 3a who had comorbidities and patients with CKD stage 3b incurred similar monthly costs associated with health insurance claims (Figure 6c)116. Those with CKD stage 4–5, however, incurred more than double these costs (P < 0.05). Patients’ dialysis status was not specified in the study publication, but a breakdown of costs was reported. Later-stage CKD was associated with higher inpatient, outpatient, and professional services costs than CKD stage 3a or 3b, whereas the highest prescription costs were incurred by patients with CKD stage 3b.
Several studies examining costs by CKD stage included patients who had progressed to RRT and therefore incurred additional costs117,118. Two studies were conducted in patient populations with CKD and DM in the USA. McQueen et al. found an increase in annual costs for each successive CKD stage, with the exception of CKD stage 2, which was associated with slightly lower costs than CKD stage 1. As in other studies, the largest increase was between the later disease stages, with a 71% increase in costs for stage 5 compared with stage 4 (Figure 7a)117. A similar cost increase was reported by Vupputuri et al. who compared costs between patients whose disease progressed and those who remained stable. Individuals who progressed to RRT incurred 77% higher annual medical costs than patients who remained at CKD stage 4 (Figure 7b)118. In a third study, Ariyaratne et al. examined the impact of CKD stage on patients undergoing percutaneous coronary intervention (PCI) in Australia. Direct cardiovascular costs, assessed 1 year after PCI, did not increase significantly for CKD stage 3 from stage 1–2 (AUD 4851 vs AUD 4442; 9% increase; P = 0.052), whereas patients with CKD stage 4–5, some of whom were receiving dialysis, incurred significantly higher costs than those at stage 1–2 (AUD 6958; 57% increase; P < 0.001)119.
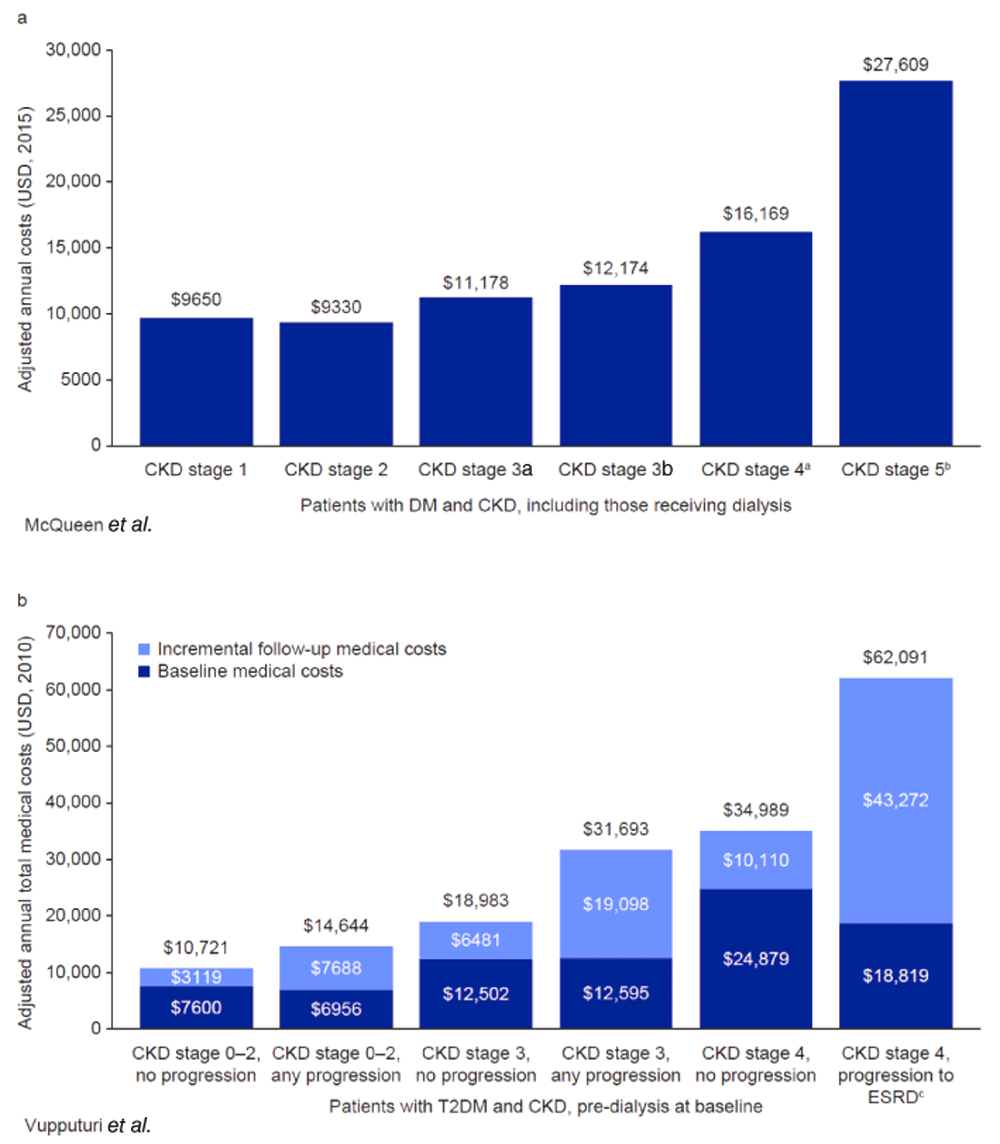
a14.4% of patients at CKD stage 4 had a procedure code for dialysis during the baseline period. b70.3% of patients at CKD stage 5 had a procedure code for dialysis during the baseline period. cESRD was defined as a requirement for dialysis or renal transplant, and was considered to be equivalent to CKD stage 5 in this study. CKD, chronic kidney disease. ESRD, end-stage renal disease. NS, nonsignificant. T2DM, type 2 diabetes mellitus. USD, US dollars.
Two studies were identified that compared costs for patients with pre-dialysis CKD with those for patients receiving dialysis (Extended data: Supplementary content 4)120–122. Eriksson et al. compared costs and resource use in Sweden between four treatment groups: patients with pre-dialysis CKD (stage 4–5), those receiving haemodialysis, those receiving peritoneal dialysis, and patients with renal transplant. Patients with pre-dialysis CKD incurred the lowest total costs and had the lowest rate of outpatient visits of any treatment group, but had a slightly higher hospitalization rate and a greater mean number of hospital days annually than those who had received a renal transplant121. Both types of dialysis incurred higher inpatient and outpatient costs than pre-dialysis CKD or renal transplant, with more than 70% of the costs associated with haemodialysis contributed by outpatient care. All types of RRT were associated with additional expenditure on drugs, compared with pre-dialysis CKD. In addition, a study by Escudero-Vilaplana et al. showed that among patients receiving erythropoietin-stimulating agents (ESAs), the monthly cost of ESA therapy was significantly higher for patients receiving peritoneal dialysis than for those with pre-dialysis CKD (stage 2–5)122.
The other studies identified in the economic burden SR did not compare patients with CKD with the general population or report data by disease severity. Data from these studies are not reported in this manuscript; however, the studies are listed in Extended data: Supplementary content 3104 to illustrate key trends and groupings in the economic evidence base for CKD. The studies that we identified reported data on the costs associated with different treatment modalities or settings26,121,123–127; on the cost of comorbidities in CKD, including DM, cardiovascular complications, and anaemia112–115,117–119,122,128–141; on hospitalization costs and rates for patients with CKD62,66,142–148; and on other costs in CKD149–155.
The studies identified in these SRs clearly illustrate the humanistic and economic impact of CKD. Patients with pre-dialysis CKD as well as patients who require RRT are likely to have worse QoL than the general population, and also incur higher healthcare costs. Several studies indicated that increasing CKD severity is associated with a gradual reduction in physical QoL; however, evidence for the impact of CKD on mental QoL was inconsistent. The economic burden SR identified strong evidence that costs and resource use are higher for patients with CKD than for the general population. Costs are especially high for patients at the most severe stage of CKD, for whom dialysis is often required. In patients with pre-dialysis CKD, costs increase incrementally with disease severity, particularly when comparing CKD stages 4–5 with stages 2–3. These findings underline the importance of early intervention in CKD to prevent patients from progressing to late-stage CKD and dialysis.
Several studies identified in these SRs report data concerning the impact of comorbidities on QoL or costs. It is likely that some of the humanistic and economic burden associated with CKD, particularly later-stage disease, is linked to comorbidities. Patients with CKD may be more likely to have comorbidities than the general population; furthermore, CKD is itself a risk factor for several complications and comorbidities, which contribute to the effect that the disease has on long-term outcomes. By examining subgroups of patients with particular comorbidities, as in the study by Wyld et al., it is possible to gain an understanding of the relative contribution of various comorbidities to overall disease burden. However, in order to study the burden of CKD specifically, analyses should be adjusted only for comorbidities that are unrelated to or are risk factors for CKD, but not comorbidities that are usually consequences of CKD. Exact differentiation between these types of comorbidities is not always possible, which can confound attempts to determine the true burden of CKD as distinct from co-existing conditions.
In this review, the impact of disease severity has been inferred almost exclusively from cross-sectional data; only one longitudinal study was identified. The economic study by Vupputuri et al. examined the impact and long-term consequences of increasing CKD severity in a patient cohort over time by comparing patients who remained at the same CKD stage and those whose disease progressed; however, the remaining economic studies and all QoL studies were cross-sectional. A longitudinal cohort study of patients with early-stage CKD could provide additional insights into the within-patient effects of CKD progression, the development of comorbidities and complications, and the competing risk of death, helping to quantify the extent to which this results in over-representation of relatively young patients or patients with few comorbidities in the later CKD stages, due to their better overall survival.
Moreover, definitions of CKD stage and grouping of patients into categories were not consistent across the evidence base, and in some cases eGFR category was used as a measure for disease severity but did not correspond exactly to the CKD stages used in other studies. Patients’ dialysis status differed between studies, as did the definition of ESRD, which was defined as the requirement for dialysis only in some studies and was expanded to include renal transplant in others. Similarly, CKD stage 5 was considered a pre-dialysis disease stage in some studies, but in others was analogous to RRT. For QoL, the use of different instruments across studies also contributed to the difficulty in drawing comparisons between different data sets. Therefore, it was not possible to strengthen our findings by performing a meta-analysis using the data identified.
These SRs identified a broad range of studies reporting QoL or cost and resource use data for patients with CKD, including the impact of treatment modality and the effect of comorbidities. In the majority of studies, however, the scope of the data reported was relatively limited or comparisons were made between two groups of patients with CKD, differentiated by other factors, such as sociodemographic characteristics or treatment; these studies were outside the scope of this review. Owing to the design of the SRs, in which restrictions were applied to publication type, population size, and QoL instrument, it is also possible that some studies of interest may not have been examined in detail. Furthermore, no assessment of risk of bias was performed.
Our findings indicate that the development and progression of CKD are associated with both a reduction in patients’ QoL and an increase in healthcare costs. Interventions and initiatives to prevent CKD progression, especially to later stages of disease and the requirement for dialysis, could improve patients’ well-being and may limit the growing economic burden of CKD. We have also identified the need for further research, particularly longitudinal studies, as well as studies that collect full information on patients’ disease history, treatment status, and comorbidities, and adjust for these factors when necessary. Such studies will be vital to quantify the impact of slowing CKD progression on the disease’s humanistic and economic burden.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: Freeman et al. Supplementary content 1 – Table S1. http://doi.org/10.6084/m9.figshare.100114766.
This project contains Table S1: Electronic search strategy.
Figshare: Freeman et al. Supplementary content 2 – Figure S1. https://doi.org/10.6084/m9.figshare.1001168318.
This project contains Figure S1: Physical and mental component summary scores with increasing severity of chronic kidney disease.
Figshare: Freeman et al. Supplementary content 3 – Table S2. http://doi.org/10.6084/m9.figshare.10011692104.
This project contains Table S2: Additional quality of life, symptom burden and cost and resource use studies identified in the systematic review.
Figshare: Freeman et al. Supplementary content 4 – Table S3. https://doi.org/10.6084/m9.figshare.10011710120.
This project contains Table S3: Summary of studies that compared costs for patients with chronic kidney disease by renal replacement therapy status.
Figshare: Freeman et al. Supplementary content 5 – Protocol. https://doi.org/10.6084/m9.figshare.11112578156.
This project contains the protocol for this systematic review.
Figshare: PRISMA checklist for ‘Humanistic burden and economic impact of chronic kidney disease: a systematic literature review’. http://doi.org/10.6084/m9.figshare.10011893157.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nephrology, outcomes of care
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: chronic kidney disease, acute kidney disease
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oncology, autoimmune diseases, cardiovascular diseases, chronic kidney disease
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 24 Dec 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)