Keywords
Cassia, Cassia fistula, Constipation, Pediatric gastroenterology
Cassia, Cassia fistula, Constipation, Pediatric gastroenterology
Typing mistakes have been corrected.
See the author's detailed response to the review by Farhad Shokraneh
See the author's detailed response to the review by Massimo Bellini
See the author's detailed response to the review by Sara Naqvi
Constipation is a clinical disorder attributed to ineffectual colonic impulsion and/or increased resistance to the proliferation of colonic matters1. Approximately 20% of the world population suffers from chronic constipation2. It is one of the most common pediatric problems3. It was found to be the second most stated disorder in the field of pediatric gastroenterology. Treatment costs for children with constipation will be around three times higher than children without constipation in the United States4. African American children, particularly girls, are greatly affected by constipation, which has been associated with poor hygiene conditions5.
Commonly, constipation is treated by Cisapride in children6, other treatments include polyethylene glycol 3350 and lactulose, however polyethylene glycol 3350 has been found to be more effective7. Supplemented and non-supplemented yogurt helps in reducing abdominal pain and to enhance defecation frequency8. It has been observed that different species of Cassia act effective as a laxative such as Cassia fistula, Cassia alata, and Cassia augustifolia9–11. The genus Cassia is well known in alternative medicine as hepatoprotective12, laxative, and in the treatment of ringworm infection13, skin diseases14 and leprosy15. It has many pharmacological properties including acting as a hypolipidemic agent16, anti-microbial17, anti-fungal18, and anti-cancer agent19. Genus Cassia contains a number of bioactive compounds such as anthraquinone20, tannin21, coumarins22, triterpene, volatile oil23, phenolic glycoside24, flavonoids25 from different parts of the plant. Different species of Cassia possess laxative properties due to various anthraquinone derivative such as aloe-emodin, rhein, chrysophanol and chrysoobtusin. In this review, we systematically assessed the laxative potential of different species of Cassia.
A systematic literature search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Using the keywords (Senna AND Laxatives AND Clinical trial) (Cassia AND Laxatives AND Clinical trial) (Cassia AND Clinical trial) (Senna AND Clinical trial) and publication range from 01 January 1960 till 31 December 2018 for identification of the records. Table 1 shows the search strategy for PubMed Central. During screening of the records only full-length open access articles were considered. Abstract only or closed access articles were excluded11. Only articles involving children aged between 2–15 were included. All review articles, in-vivo studies and those >10 years from the search date were excluded. A preliminary search of the PubMed, CNKI, Scopus, Web of Science, Google Scholar and PsycINFO databases and digital archive such as PubMed Central, yielded 2207 papers published in English from the last ten years. Duplicate and irrelevant articles were removed (n=2203). One article was further removed during screening due to closed access (n=3). One publication was removed because the article did not meet the eligibility criteria (n=2). A PRISMA Flow Diagram is given in Figure 1.
Identification of articles were performed at level 1 using the search strategy as mentioned in Table 1. Duplicate articles, irrelevant articles such as polyherbal formulation, review articles, or any article other than Cassia or Senna were removed at level 2. Only four4 articles were identified as being relevant. One record was excluded due to not being a full text article. Abstracts were being reviewed for the following inclusion and exclusion criteria at level 4 and one article was removed for not meeting the eligibility criteria i.e. Randomized, clinical trial on Constipation, full-length open access articles, Pediatric Functional Constipation (age range: 2–15 years).
Types of studies. The author has selected studies of randomized open label, prospective, controlled, parallel-group clinical trial for meta-analysis. Baseline characteristics of randomized trials of studies included on pediatric functional constipation are presented in Table 4. Characteristics of the studies included are mentioned in Table 5.
Types of participants. The author included studies involving patients (aged 2–15 years) with Functional constipation. The diagnosis of Functional constipation was according to according to the Rome III criteria26. Inclusion and exclusion criteria were based on Study design, participants, intervention, outcome (SPIO) criteria and indicated in Table 2.
Types of interventions. Included studies were focused on the role Cassia in the treatment of Functional constipation. Unfortunately, there were only two studies identified.
Types of outcomes. Eligible studies included consisted of the following outcomes: improvement in the episodes of fecal incontinence per week, improvement in the episodes of retentive posturing per week, improvement in the average of severity of pain of defecation (by VAS), improvement in defecation frequency per week, patient’s drug compliance and improvement in the average of consistency of stool defecated (by VAS).
Methodological quality assessment was made on the basis of following criteria. 1) Aims and Hypothesis clearly defined, adequate sample representation, patient care quality, ethical approval protocol, outcomes assessment, validity and reliability of outcome measure, attempt to blind researcher, follow-up, appropriate statistical analysis and missing data reported. Ten item defined evaluation of methodological quality (MQ) is presented in Figure 2. Risk of Bias were assessed using Cochrane collaboration’s tool on the basis of the following criteria such as selection bias, performance bias, attrition bias, reporting bias and miscellaneous. Cochrane Collaboration’s tool for assessing the risk of bias was used and the results are presented in Table 627.
The following data were extracted according to study characteristics (e.g., first author, year of publication, search dates, and number of included studies), patient characteristics (functional constipated children, aged between 2–15), sample size, study type (e.g., Randomized open label, prospective, controlled, parallel-group clinical trial study), randomization methods (e.g., “systematic randomization and simple randomization”) and outcome measures/variables (e.g., improvement in defecation frequency per week, improvement in the episodes of fecal incontinence per week, improvement in the episodes of retentive posturing per week, improvement in the average of severity of pain of defecation (by VAS), improvement in the average of consistency of stool defecated (by VAS) and patient’s drug compliance). Data extraction was performed by Muhammad Shazad Aslam. Transcripts were analysed, coded and data was extracted using the demo version of qualitative data analysis software Atlas.ti 8.0. Table 3 represent all the data that was extracted. All the meta-data are available as Dataset 1.
Meta-analysis was conducted using the Review Manager (RevMan) 5.3 software28. The summary measures were reported as odds ratios (ORs) or as a standard mean difference (SMD) with 95% confidence intervals (CI). The presence of heterogeneity among trials was assessed using the Chi-square test, and the extent of the inconsistency was measured by I2 statistics. Output file from RevMan is available as Underlying data29.
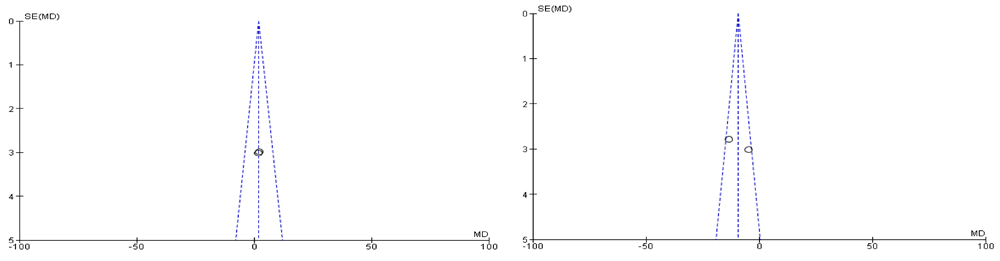
Both of the selected studies were not blinded during intervention and outcome assessment that will result in performance bias and detection bias respectively. These biases occur where the investigators know about the participant's treatment group. Performance bias can also refer to the fact that participants can change their responses or behaviour if they know which group they are allocated in. Blinding of outcome assessment may decrease the risk of the investigator or participant being aware of the treatment that a patient is receiving. If the participants and the caregivers are aware of the intervention and outcome that may affect the behavior of the participants, these behavioral changes may affect the performance of the treatment. Clinical trials on adults were also excluded, such as a randomized clinical trial of a phytotherapeutic compound containing Pimpinella anisum, Foeniculum vulgare, Sambucus nigra, and Cassia augustifolia for chronic constipation9. Results of both included studies were non-significant when comparing their baseline characteristics of pediatric functional constipation as presented in Table 5. During analysis of study characteristics, it was found that both of studies demonstrated Cassia fistula is helping to treat constipation among the children as shown in Table 3, but there is a risk of bias according to Cochrane Collaboration’s tool (Table 6). Moreover, both studies found were from one country (Iran). During a methodological assessment, many flaws were identified such as inadequate patient care, attempt to blind the researcher and missing data (Figure 2). During meta-analysis, the comparison was made before and after treatment among different variables such as defaecation, fecal incontinence, retentive posturing, the severity of pain, and consistency of stool. All the variable (before and after treatment) were found to be symmetrical when plotted on a funnel plot as shown in Figure 4, Figure 6, Figure 8, Figure 10, Figure 12, and Figure 14 respectively. The overall effect for some variables is statistically insignificant (P=0.11, P=0.49, P=0.24) such as fecal incontinence, retentive posturing, and acceptance, tolerance respectively. High heterogeneity was found in two variables i.e severity of pain (90%) and consistency of stool (77%). All the forest plot of defaecation, fecal incontinence, retentive posturing, severity of pain, consistency of stool and acceptance and tolerance are represented in Figure 3, Figure 5, Figure 7, Figure 9, Figure 11 and Figure 13 respectively.
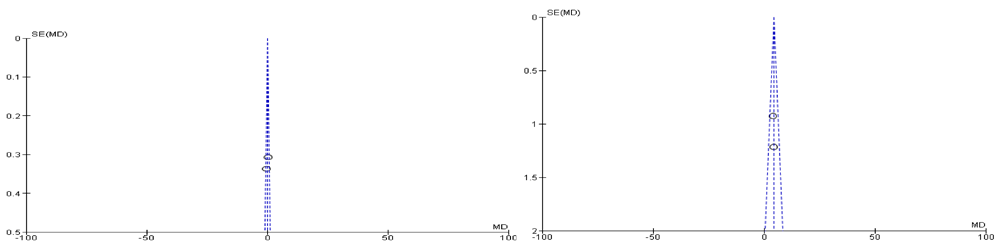
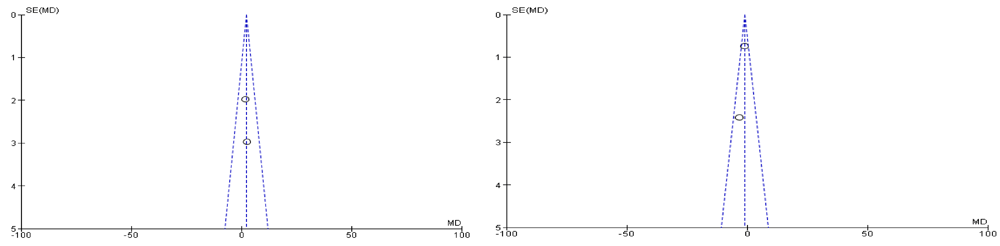
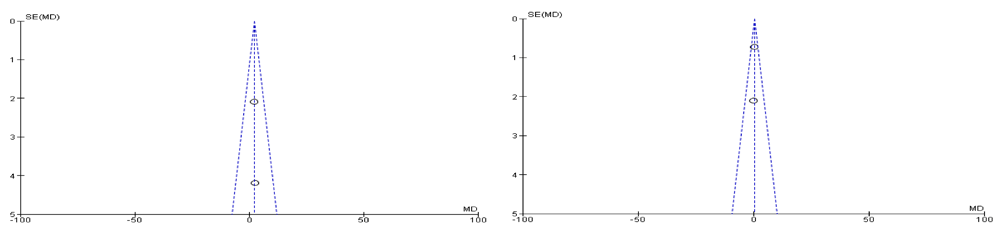
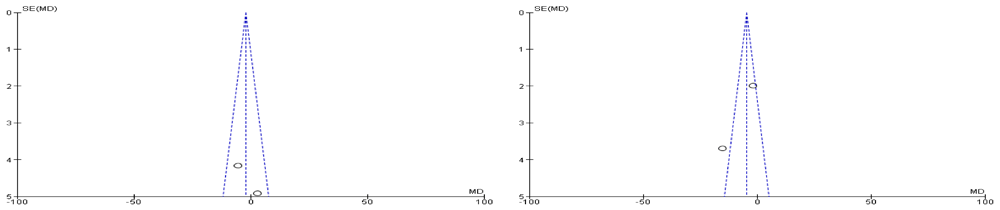
After evaluation of results, it was found that Cassia fistula was not completely effective. It was partly effective in reducing the pain and consistency of stool during constipation. However, these results cannot be generalized among all population. A well designed, expert validated protocol is required in the future. There is a need to develop an instrument that will be free from bias. Moreover, the results cannot be generalized among all species of Cassia as the studies available are only for one species. There is a need to isolate identified bioactive compounds from different species of Cassia and evaluate the effect of different factors such as duration of constipation, defecation, incontinence or retentive posturing under clinical trial.
Underlying data. Open Science Framework: Application of genus Cassia in the treatment of Constipation: A systematic review. https://doi.org/10.17605/OSF.IO/PKR4N29
This project contains the following underlying data:
Open Science Framework: PRISMA diagram and flowchart for the study “Application of genus Cassia in the treatment of constipation: A systematic review”. https://doi.org/10.17605/OSF.IO/PKR4N29
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products, Phytochemistry, and Gut motility.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gastroenterology: Functional Digestive Disorders.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
No
Are the conclusions drawn adequately supported by the results presented in the review?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Evidence synthesis, Systematic review, Overview, Scoping review, Rapid review, Randomized controlled trials, Open data, Open access, Open source, Medical journalism, Peer-review, Open source, Outcomes, Interventions, Cochrane
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gastroenterology: Functional Digestive Disorders.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 08 Mar 21 |
read | read | |
|
Version 1 05 Mar 19 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)