Keywords
Aedes, Anopheles, Culex, chikungunya, Rift Valley fever, dengue fever
Aedes, Anopheles, Culex, chikungunya, Rift Valley fever, dengue fever
The names of the pathogens have been rewritten based on the general rule, typos have been corrected accordingly and the conclusion has been strengthened as recommended.
See the authors' detailed response to the review by Laban Njoroge
See the authors' detailed response to the review by Nicholas Johnson
Mosquitoes are vectors responsible for transmission of numerous pathogens causing diseases such as, malaria, lymphatic filariasis, avian malaria and arboviruses including dengue virus, chikungunya virus, yellow fever virus, West Nile virus, and Zika virus (Charrel et al., 2007; Semenza, 2014). Africa is one of the major hosts of mosquitoes responsible for mosquito-borne viruses (Braack et al., 2018) that are of great medical importance and contribute to the current global public health threat (Enserink, 2007; Gubler, 2002; Higgs, 2014). Seasonal and environmental changes play a role in the global distribution of mosquito species and the arboviruses they transmit (Anyamba et al., 2001; Hasnan et al., 2016). The global spread of vector-borne diseases has resulted in multiple calls on nations to enhance surveillance of emerging arboviruses that requires understanding of the species composition and distribution of potential mosquito vectors (Grout et al., 2017; Kollars et al., 2016).
In the recent past, there has been an increasing spread of mosquito-borne viruses such as chikungunya virus, dengue virus and Rift Valley fever virus in Kenya, thus prompting a need for further research (Johnson et al., 1982; Konongoi et al., 2018). The available literature on mosquitoes in Kenya mainly addresses aspects of morphological identification of mosquito vectors and limited molecular characterization (Lutomiah et al., 2013; Mwangangi et al., 2013). Despite mosquitoes being a key public health challenge in Kenya, little is known about their species diversity and distribution along different ecological zones such as the Kenyan coast and Kenya’s capital city. Subsequently, population genetic studies on mosquito vectors in Kenya have focused on the Anopheles genus because of its significance in endemic malaria transmission (Chen et al., 2006; Chen et al., 2004; Lukindu et al., 2018). In addition, most of the studies on mosquito vector composition and diversity are based on mosquitoes confined to a single habitat or with a limited habitat range (Ajamma et al., 2016a; Muturi et al., 2006). The species composition and distribution of Anopheline mosquitoes in Kenya, particularly along the Kenyan coast, have broadly been reported over that of Culicine mosquitoes (Mbogo et al., 2003; Midega et al., 2007; Midega et al., 2010). Moreover, little has been documented on the species composition and diversity of all mosquito groups by use of molecular markers. As such, understanding the species composition and diversity patterns of the suggested vectors is pivotal to the judicious deployment of existing vector control strategies and the development of new effective vector control interventions (Kraemer et al., 2016).
In this study, we employed molecular genetic techniques, involving PCR and sequencing of cytochrome oxidase subunit 1 (CO1) gene to identify and characterize mosquito species in Nairobi, Kisumu and Kilifi Counties in Kenya.
This study was carried out at Nairobi, Kilifi and Kisumu Counties in Kenya. Kilifi and Kisumu regions were chosen purposively due to their high abundance of mosquito vectors (WHO, 2017) and vector-borne disease burden, while Nairobi region was selected because it’s a major international and domestic destination for both humans and parasites (Wesolowski et al., 2012). Two sampling sites were randomly selected from each of the three regions as follows: Kisumu; Ahero and Kisumu town, Kilifi; Kilifi town and Mazingira Park and Nairobi; Nairobi city centre, and Northern Bypass (Figure 1).
The trapping of mosquitoes was carried out in the respective counties during the dry season (January–February 2018) and wet season (March–April 2018). The captures were conducted day and night using the Pyrethrum Spray Catch (PSC) method as used by (Ndiath et al., 2011). The specimens were adult mosquitoes, which were morphologically sorted in the field into their respective genera, and transported in liquid nitrogen to the laboratory for further molecular analysis. A total of 2,438 adult mosquitoes were collected. Of these, 894, 824 and 720 adult mosquito samples were collected in Nairobi, Kisumu and Kilifi respectively. From the overall collection, 300 hundred mosquitoes per county were randomly selected for PCR. A total of 25 sequences per study region were used for phylogenetic and genetic diversity analysis as described in an earlier study (Hale et al., 2012).
Total genomic DNA was extracted from whole body of individual mosquitoes using the Collins’ protocol (Collins et al., 1987) with minor modifications. A DNA homogenizing buffer (containing 0.1 M NaCl, 0.2 M sucrose, 0.01 M EDTA and 0.03 M Tris pH 8) was mixed with a lysis buffer (containing 0.25 M EDTA, 2.5% w/v SDS and 0.5 M Tris pH 9.2) in the ratio of 4:1 to make up the grinding buffer (GB). Each mosquito was homogenized in 100 цL of the GB, using a hand-held pestle homogenizer and incubated for 30 min at 55°C. Into each sample, 14 цL of 8 M potassium acetate (KAc), a deproteinating reagent was added and then incubated for 30 min at room temperature before centrifuging at 13,000 rpm for 15 min to get the supernatant that contained the nucleic acid component. 95% ethanol was used to precipitate the genomic DNA. Centrifugation at 13,000 rpm for 10 minutes was done to obtain the nucleic acid pellet. This was followed by a washing step using 70% ethanol. The DNA pellet was suspended in 100 μL of T.E buffer pH 7.2 and stored at –20°C awaiting subsequent experimental procedures.
The primer set; Forward (LCO1490_GGTCAACAAATCATAAAGATATTGG) and Reverse (HCO2198 TAAACTTCAGGGTGACCAAAAAATCA) synthesized by Macrogen (OG180803-187) and previously published by Folmer et al. were used in molecular identification of the mosquito species (Folmer et al., 1994). In a 10 µL PCR reaction volume, the PCR mix consisted of 2 µL 1× HOT FIREPol® Eva Green mix (Solis BioDyne, Tartu, Estonia) catalogue number 08-31-00008, 6 µL of nuclease-free water, 0.5 picomoles of each primer and 1 µL of the DNA template. The fragments were amplified using applied biosystems ProFlex SN 297802057 thermocycler under the following cycling parameters; initial denaturation for 15 min at 95°C, followed by 35 cycles of denaturation at 95°C for 30 sec, annealing at 50°C (Anopheles, Aedes, Culex) for 30 sec, and extension at 72°C for 30 sec, and a final extension at 72°C for 7 min. The PCR products from the amplification of the mitochondrial cytochrome c oxidase 1 (CO1) region of the mosquito after purification using QIAquick® gel extraction kit catalogue number 28706, were shipped for sequencing at Macrogen Inc., South Korea.
Resultant mitochondrial cytochrome c oxidase 1 (CO1) sequence chromatograms were edited and visualized using Chromas Lite version 2.6.5. The sequences were deposited in GenBank and accession numbers assigned accordingly. Consensus sequences were aligned using ClustalX version 2 (Thompson et al., 1997), and visualized using Seaview version 4.7 (Gouy et al., 2010). Unique sequences (haplotypes) were identified using DnaSP version 6 (Librado & Rozas, 2009). Sequence polymorphisms were identified using DnaSP and visualized using Jalview version 2.10.5 (Waterhouse et al., 2009). DNA sequence divergence was analysed using DnaSP. These unique sequences were compared with reference sequences from other parts of the world, selected to represent the Aedes, Anopheles and Culex genera previously reported and available from GenBank (Benson et al., 2011). Other sequences similar to the study sequences in GenBank obtained using the Blastn algorithm were also included in the analysis. Multiple alignment and comparison of the study sequences and GenBank references were performed using ClustalX. Phylogenetic and molecular evolutionary analyses were conducted using Software for Molecular Evolutionary Genetics (MEGA7) (Kumar et al., 2016). Phylogenetic trees were constructed using the maximum likelihood (ML) method rooted using Lutzomyia longipalpis. The phylogenetic trees were estimated using the best-fit general time-reversible (GTR) model of nucleotide substitution with gamma-distributed rate variation among sites. Bootstrap resampling process (1000 replications) was employed to assess the robustness of individual nodes of phylogeny (only >50% were indicated). The resultant tree was visualized using Dendroscope version 3 (Huson & Scornavacca, 2012).
From each study site, 25 CO1 gene amplicons were sequenced for phylogenetic analysis. In total, 14 haplotypes belonging to genera Aedes, 9 haplotypes belonging to genera Anopheles and 12 haplotypes belonging to genera Culex were identified through CO1 sequence analysis. These sequences were deposited in GenBank and assigned accession numbers (Table 1). Sequence analysis revealed a unique Anopheles haplotype (GenBank accession number, MK300230) (Figure 3). Subsequently, haplotypes of Anopheles gambiae, Anopheles funestus, Aedes cumminsii, Aedes aegypti, Culex pipiens and Culex sitiens were found to be distributed across Kilifi, Kisumu and Nairobi mosquito populations (Table 1).
Diversity indices for the three populations, based on sequenced results were calculated as shown in (Table 2). Average number nucleotide differences (k), nucleotide diversity Pi (π) and haplotype diversity (Hd) varied among the species (Table 2).
Phylogenetic analysis of fourteen (14) Aedes haplotypes from Kilifi and Nairobi with similar sequences based on Blastn (NCBI) search and sequences of known Aedes identity revealed that study Aedes haplotype Accession number MK300225 clustered with Aedes cumminsii (Figure 2). Study haplotypes Accession number; MK300216, MK300217, MK300218, MK300219, MK300220, MK300221, MK300222, MK300223, MK300224, MK300226, MK300227, MK300228 and MK300229 clustered with Aedes aegypti that has been previously identified in France (Accession number HQ688297.1). Significantly, they also clustered with Aedes aegypti (Accession number KX420485.1, KX420429.1 and KU380400.1) previously reported in Nyanza-Kisumu, Kenya (Figure 2). Genetic divergence between study Aedes haplotypes identified in Kilifi and Nairobi and Aedes species they clustered with (sequences of known species obtained from GenBank) was variable (Table 3). There was limited divergence between Aedes aegypti (Accession number KX420485) that has previously been identified in Nyanza-Kisumu, Kenya and study haplotypes MK300216, MK300222, MK300218 and MK300221. Aedes aegypti (Accession number KU380400.1) that has been reported in Nyanza-Kisumu, Kenya before showed limited divergence with study haplotype MK300217. Limited divergence was also identified between haplotype MK300224 and Aedes aegypti (Accession number HQ688297.1) that has been characterized in France. Greater divergence and heterogeneity was observed between Aedes aegypti and study haplotypes MK300225, MK300219 and MK300229. Study haplotypes MK300216, MK300220, MK300223, MK300227 and MK300228 formed a distinct clade with other Aedes aegypti of known identity (Figure 2).

The scale represents the number of differences between sequences (0.02=2%). The gamma correction for rate heterogeneity was 0.1963. The analysis involved 46 nucleotide sequences. There were a total of 657 positions in the final dataset.
Phylogenetic analysis of haplotypes with similar sequences to those of known identity showed a clustering of study Anopheles haplotype MK300231 and MK300232 with Anopheles funestus. Notably, they also clustered with Anopheles funestus (Accession number MH299888.1 and KU380404.1) that has been reported in Kilifi and Baringo counties in Kenya respectively (Figure 3). Study haplotype MK300233, MK300234, MK300235, MK300236, MK300237 and MK300238 clustered with Anopheles gambiae previously isolated in Uganda (Accession number MG753695.1, MG753730.1 and MG753745.1) (Figure 3). Anopheles haplotype MK300230 formed its own distinct clade. This study haplotype MK300230 may be a new species or novel haplotype not yet described (Figure 3). Genetic divergence between Anopheles haplotypes identified in Kisumu, Kilifi, Nairobi and Anopheles species from GenBank they clustered with was variable in some haplotypes while others were not variable (Table 4). There was very limited divergence and heterogeneity between Anopheles funestus and study haplotype MK300231 and MK300232. There was no divergence between Anopheles gambiae (Accession number DQ792577.1 and MG753695.1) and study haplotype MK300234. Anopheles gambiae (Accession number MG753695.1) has been identified in Uganda before. Study haplotypes MK300235, MK300233, MK300238, MK300236 and MK300237 showed limited divergence with Anopheles gambiae.

The gamma correction for rate heterogeneity was 0.1647. The analysis involved 57 nucleotide sequences. There were a total of 658 positions in the final dataset.
From the phylogenetic analysis, we further established that 12 Culex haplotypes from Kilifi, Kisumu and Nairobi, and similar sequences of known identity based on Blastn (NCBI) showed a clustering of study haplotype MK300240, MK300242, MK300246, MK300247, MK300248, MK300249 and MK300250 with Culex pipiens that have been identified in different regions of the world. Importantly, they clustered with Culex pipiens that has previously been identified in Nyanza-Kisumu, Kenya (Accession number KU380381.1, KU380372.1) (Figure 4). Study haplotypes MK300239, MK300241, MK300243, MK300244, MK300245 clustered with Culex sitiens that was earlier identified in Australia (Accession number MG712559.1) (Figure 4). Genetic divergence between Culex haplotypes identified in Kisumu, Kilifi, Nairobi and reference Culex species was slightly variable in some species, while other species showed no divergence (Table 5).
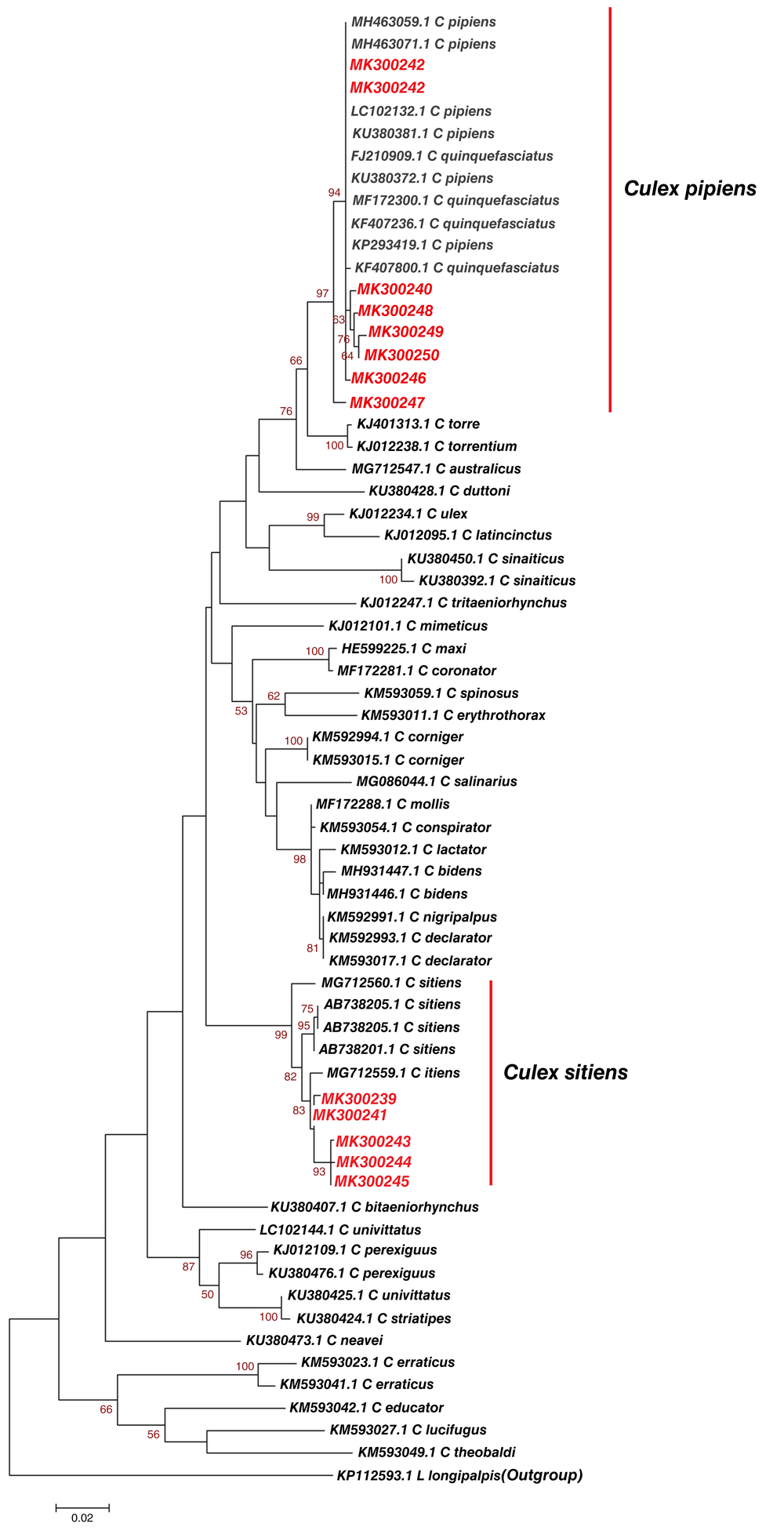
The gamma correction for rate heterogeneity was 0.1790. The analysis involved 62 nucleotide sequences. There were a total of 658 positions in the final dataset.
This study identified Aedes aegypti in both Kilifi and Nairobi populations and Aedes cumminsii in the Kilifi population only. Anopheles gambiae was identified in both Kisumu and Nairobi population whereas Anopheles funestus was identified in Kisumu population only. A potentially novel Anopheles haplotype MK300230 was identified in Kilifi population. Culex pipiens was identified in all the three populations; Kisumu, Nairobi and Kilifi while Culex sitiens was only identified in the Kilifi population. The greatest diversity was in the genus Aedes that has 14 haplotypes, followed by Culex 12 and Anopheles 9, this is consistent with other studies looking at mosquito diversity in different ecological regions in Kenya (Mwangangi et al., 2012). Similarly, out of the 35 mosquitoes haplotypes identified in Kilifi, Nairobi and Kisumu regions, one Culex haplotype MK300242 from this study has been previously reported in Kisumu-Nyanza in Kenya and in Portugal (Ajamma et al., 2016b; Mixão et al., 2016), and one Anopheles haplotype MK300234 in Uganda (Lukindu et al., 2018). The Kilifi mosquito population had the greatest diversity and abundance of mosquito species, possibly due to its geographical position, human activities, and natural climatic conditions.
Aedes cumminsii has been morphologically identified in Kenya before (Mwangangi et al., 2006), however, this study reports the first molecular characterization of Aedes cumminsii in Kenya. Aedes haplotypes between Kilifi and Nairobi populations were divergent based on nucleotide diversity tests; this could be due to different climatic zones. Thus, diversity in vector haplotypes plays an important role in vector control and management practices and epidemiology of vector borne diseases (Murugan et al., 2016). Phylogenetic analysis showed presence of two Aedes species that is Aedes cumminsii and Aedes aegypti, in Kilifi, while Nairobi had only Aedes aegypti (Figure 2 and Table 1). This study has identified 4 different Aedes aegypti haplotypes in Nairobi. Previous studies on survey of mosquito composition in Nairobi have indicated low percentage of Aedes mosquito (Kinuthia et al., 2017). There is therefore increased diversity in Aedes aegypti species from Nairobi; diversity and spread of Aedes aegypti has been attributed to the increase in arboviral infections (Woolhouse et al., 1997). The diversity of Aedes aegypti in Nairobi could be the result of high population density (Gubler & Clark, 1995), poor sanitation and waste disposal as well as water management (Monath, 1994). The Kilifi population had genetically diverse forms of Aedes aegypti (Table 2). Aedes aegypti is widespread on the Kenyan coast (McDonald, 1977; Teesdale, 1955). It is the principal vector of dengue virus, chikungunya, and urban yellow fever virus (Reiter, 2010), and it was predominant in the Kilifi samples. This may contribute to their high susceptibility to dengue-outbreak reported in the region (Baba et al., 2016; Chepkorir et al., 2014). Secondly, factors relating to availability of breeding sites, temperature or altitudinal differences may have influenced the diversity patterns of Aedes aegypti in Kilifi (Barrera et al., 2011). Evidence of high diversity of Aedes aegypti in Kilifi also means that the Kenyan coast is consistently at higher risk of yellow fever transmission (Agha et al., 2017). Kilifi lies in between Malindi and Mombasa cities which are popular destinations for international tourism as well as maritime industry, and where Aedes aegypti is widespread (Ngugi et al., 2017). Human trade and travel may bolster movement of Aedes aegypti (Powell & Tabachnick, 2013) and contribute to diversity of the species. In addition, invasion risk related to human travel has become far more severe (Egizi et al., 2016; Wilder-Smith & Gubler, 2008). Phylogenetic relationship between Aedes species from this study and other Aedes species of known identity from GenBank showed clustering with Aedes cumminsii and Aedes aegypti at a high bootstrap value (>90%) at the defining node on the phylogenetic tree (Figure 2). However, genetic diversity between Aedes species from this study and those of known identity from GenBank was variable (Table 3).
Anopheles species were distributed across the three study populations Kisumu, Nairobi and Kilifi (Table 1). Anopheles species between Kilifi, Kisumu and Nairobi populations were highly divergent as analyzed using molecular markers. Nairobi had only one haplotype of Anopheles gambiae (Table 1). Anopheles mosquitoes have also been reported in places where malaria has been eradicated and also in malaria non endemic regions thus increasing the risk of reintroduction of malaria as well as spreading of malaria to new areas (Martens & Hall, 2000). Other than transmitting malaria, Anopheles mosquitoes have been indicated as carriers of arboviruses including West Nile virus and Japanese encephalitis (Thenmozhi et al., 2006), as well as viruses that cause o’nyong-nyong and chikungunya fevers (Vanlandingham et al., 2005). This study has indicated higher diversity of Anopheles haplotypes in the Kisumu population, having detected Anopheles gambiae and Anopheles funestus (Table 2). High diversity of Anopheles vector is a key feature for consideration in Anopheles management and has been associated with the rise in malaria transmission (Loaiza et al., 2012). The low diversity of Anopheles species in Kilifi and Nairobi may be attributed to the Great Rift Valley and, high-elevation mountains in western Kenya. The vast arid area in the east of the Great Rift Valley inhibits human settlement, thus restricting Anopheles funestus gene flow between coastal and western Kenya (Lukindu et al., 2018). Anopheles funestus is closely associated with human dwellings and therefore plays an important role in the transmission of malaria (Kweka et al., 2013). Anopheles gambiae haplotypes in Kisumu were diverse, this is consistent with other studies that have reported a high genetic diversity of Anopheles gambiae in Kisumu Kenya (Chen et al., 2004). Phylogenetic analysis (Figure 3) and nucleotide diversity tests (Table 4) showed no divergence between Kisumu Anopheles gambiae haplotype MK300234 with Anopheles gambiae MG753695.1, used as reference that was previously isolated in Uganda (Lukindu et al., 2018). This indicates the presence of genetically identical Anopheles gambiae between Kenya and Uganda which could be attributed to cross-border migration, or retention of shared ancestral polymorphism. Therefore, this could suggest that, these species share the same ecological niche or ancestral divergence. Anopheles gambiae (s.s.) (formerly Anopheles gambiae S-form) is a main vector of malaria in sub-Saharan Africa, where 90% of an estimated 445,000 malaria deaths worldwide occurred in 2016 (CDC - Malaria - About Malaria - Disease). Presence of both Anopheles gambiae and Anopheles funestus in Kisumu suggest that the area is still at high risk of malaria transmission. This study has identified a potentially new haplotype of Anopheles species MK300230 in Kilifi (Figure 3). Through molecular techniques new haplotypes of Anopheles species are continually being identified; for instance, new species of Anopheles nuneztovari have been identified in Brazil (Scarpassa et al., 2016).
Culex pipiens was distributed across Kilifi, Kisumu and Nairobi population while Culex sitiens was only identified in Kilifi population (Table 1). Culicidae is a large and abundant group that occurs throughout temperate and tropical regions of the world, as well as the peri Arctic Circle (Schäfer & Lundström, 2001). Culex mosquitos are an important vector of the zoonotic infection filariasis. Human filariasis infection is a major public health concern. Approximately 57% of those at risk of infection is in the South-East Asia Region and 37% in the African Region (“WHO | Epidemiology,” 2018). Although Culex pipiens is ornithophilic it can also feed on humans and mammals (Reisen et al., 1990) and thus capable to transmit West Nile virus to humans. Culex pipiens (Linnaeus) has been identified as the primary vector of West Nile virus (Turell et al., 2000). Kenyan strain of Culex pipiens has been confirmed to be capable of transmitting West Nile virus and its circulation among humans in Kenya has been detected (Lutomiah et al., 2011; Morrill et al., 1991). Therefore, the distribution of Culex pipiens across Kilifi, Nairobi and Kisumu could increase the risk of West Nile virus transmissions/outbreaks in most parts of Kenya. Culex pipiens haplotype MK300242 was identified in both Kilifi and Kisumu population (Figure 4). This study reports distribution of identical mosquito vector species between populations. Phylogenetic analysis revealed Culex pipiens haplotype MK300242 from this study showed no divergence to the Culex pipiens sequences LC102132.1 from Portugal and KU380381.1, KU380372.1 from Nyanza Kenya (Table 5). This study identified Culex sitiens in the Kilifi population only, Culex sitiens has been found to tolerate saline waters, in Oman it has been successfully isolated from brackish water (Roberts, 1996). Consequently, parasites such as Microsporidium, Amblyospora have been isolated from Culex sitiens mosquito in Coastal Kenya (Sabwa et al., 1984).
Results from this study demonstrate that mosquito vectors that have been associated to arboviral pathogens are distributed across Kilifi, Nairobi and Kisumu counties. 35 haplotypes belonging to genus Anopheles, Culex and Aedes have been identified, genetic diversity of this haplotypes varies with some genus recording high diversity where’s others had low diversity. A potentially new haplotype belonging to Anopheles genus has been identified. This implies further research on genetic characterization of mosquitoes in Kenya for an appropriate vector control and management program across the whole country.
Culicidae cytochrome c oxidase subunit 1 (COI) gene, partial cds; mitochondrial. PopSet 1573759763: https://www.ncbi.nlm.nih.gov/popset/1573759763?report=genbank. Accession numbers MK300216 – MK300250
We thank International Centre of Insect Physiology and Ecology (ICIPE) for hosting the research work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical entomology; Genetics
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Zoonotic viruses
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Lutomiah J, Bast J, Clark J, Richardson J, et al.: Abundance, diversity, and distribution of mosquito vectors in selected ecological regions of Kenya: public health implications.J Vector Ecol. 2013; 38 (1): 134-42 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: I am a mosquito taxonomist and ecologist. I am not an expert in sequencing and phylogenetic analyses and therefore that bit may require another expert.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Zoonotic viruses
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 24 Sep 19 |
read | read | |
|
Version 1 06 Mar 19 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)