Keywords
Burden, smoking, alcohol, diet, physical activity, healthcare cost
Burden, smoking, alcohol, diet, physical activity, healthcare cost
Smoking, unhealthy alcohol consumption, poor diet and physical inactivity are leading risk factors for morbidity and mortality worldwide1. Despite this knowledge, prevalence of these risk factors remains high and reduction efforts may be hindered by failure to understand the full human and cost burdens these risk factors impose on societies2. In an era of increasing health care expenditure most political focus has been on payments for services and the growing impacts of diagnostic and therapeutic technologies. There is also a need to consider costs resulting from upstream health behaviours, and how these have changed over time, to help prioritize public health strategies and support public health decision makers. These relationships are likely to be complex because of some conflicting trends in the prevalence of health behaviours3,4.
While health behaviours have a leading role in morbidity and mortality, it is also recognized that there is uneven distribution of health across socioeconomic position (i.e., social and economic factors that influence what position individuals hold within the structure of a society5). Canadian research indicates that individuals with lower socioeconomic position tend to be less healthy than those who enjoy greater educational, income and occupational advantages6–9. As with health behaviour-attributable health care use, evidence of the economic cost of these health disparities helps us understand the health and financial benefits of reducing the gap6.
We sought to estimate the economic burden attributable to four health behaviour risk factors (smoking, unhealthy alcohol consumption, poor diet and physical inactivity), how these have changed over time, and how they interact with socioeconomic position. The study had three objectives: 1) to examine the direct healthcare costs associated with smoking, unhealthy alcohol consumption, poor diet and physical inactivity; 2) to examine the change in direct health care costs as a consequence of changes, over time, in health behaviours; and, 3) to examine the direct health care costs associated with socioeconomic position (i.e., education, family income, home ownership, and neighbourhood deprivation).
Past studies typically infer health care costs indirectly—where aggregate health care expenditure data is categorized by disease, for example. We used unique Canadian data that individually link respondents from large repeated population health surveys to comprehensive health care utilisation and cost data covering hospital and primary care sectors in Ontario. These data provide, to our knowledge, the largest and most complete population-based examination of the relationship between health behaviours and direct public healthcare costs. We believe this is the first study to measure directly how changes in health behaviours result in changes in health care use. The linked data also provide the means to assess the degree to which health costs are associated with socioeconomic inequalities.
This study was approved by the Ottawa Health Science Network Research Ethics Board. Datasets were linked using unique encoded identifiers and analysed at ICES. ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyse health care and demographic data, without consent, for health system evaluation and improvement.
We used the Ontario sample drawn from a national population health survey—the Canadian Community Health Survey (CCHS), conducted in 2003, 2005, 2007-2008, 2009-2010, 2011-2012, and 2013-2014 to develop linked and unlinked study cohorts. The CCHS is a cross-sectional survey conducted by Statistics Canada that collects data related to health determinants, health status and health care use—details of data collected and used in the analyses are provided below. The survey employs a complex multistage sampling strategy to randomly select households in each health region. Details of the survey methodology have previously been published10. A weight, which reflects the number of individuals represented in the target population, is assigned to each respondent; the target population includes individuals aged 12 years and older living in Canada’s ten provinces and three territories. Individuals living on First Nation Reserves, institutionalized residents, full-time members of the Canadian Forces and residents of certain remote areas are excluded from the survey’s sampling frame.
For the linked study cohort, the Ontario sample of the 2003, 2005, and 2007-2008 CCHS cycles provided 128,501 valid interviews. Of these respondents, a subset agreed to share and link their interview information and 101,506 were successfully linked to their provincial health card number using a deterministic and probabilistic algorithm. We included respondents aged 25 years and older if they were eligible for provincially funded health care and not pregnant at the time of survey administration. For individuals with multiple interviews, only the earliest interview was included. We excluded individuals who were lost to follow-up in the first year following their interview (i.e., they were not available at the beginning of the study). This resulted in a final cohort of 80,749 unique Ontario respondents (see Figure 1).
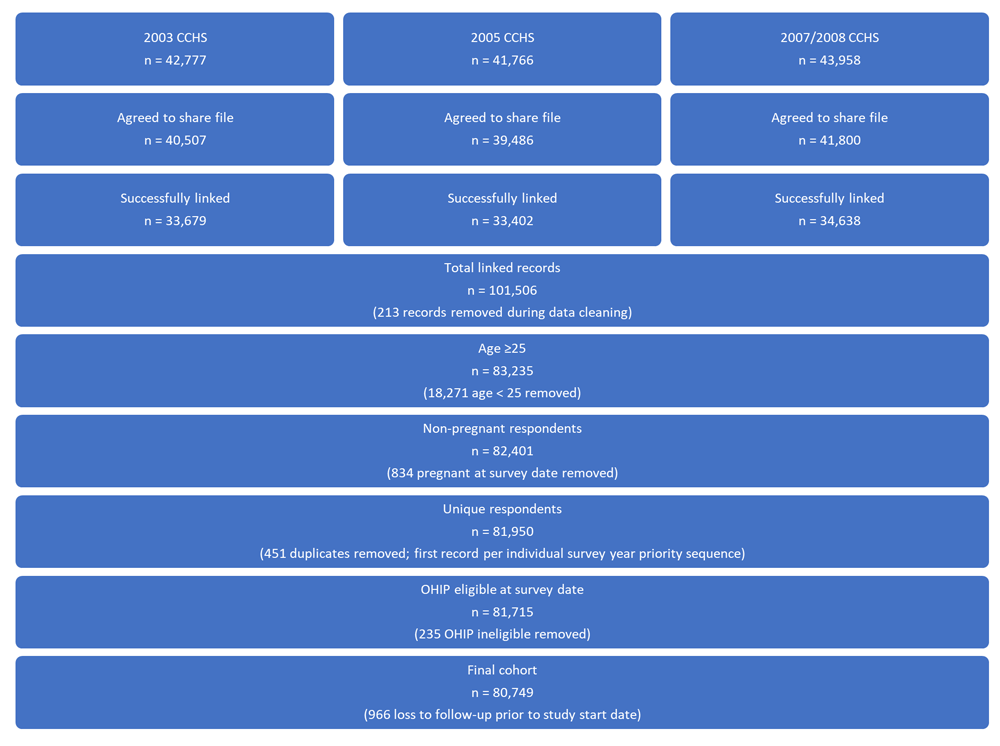
CCHS, Canadian Community Health Survey; OHIP, Ontario Health Insurance Program.
The Ontario sample of all six CCHS waves provided 200,324 valid respondents aged 25 years and older for the unlinked study cohort. This cohort was used to estimate direct health care costs by applying the multivariable model that was derived using the linked cohort CCHS data (see Model Development).
The CCHS waves were used to examine the following risk factors for their association with health care use: age, sex, four health behaviours (smoking, alcohol consumption, diet and physical activity; see Table 1), sociodemographic factors (immigrant status, education level, urban dwelling, neighbourhood deprivation, household income, home ownership, marital status), self-perceived stress, preventive health behaviour (flu vaccination), and health status indicators (body mass index, hypertension, diabetes, heart disease, cancer, history of stroke, dementia, and extent of difficulty in performing basic tasks or participating in activities).
| Behaviour | Category* | Definition |
|---|---|---|
| Smoking | Heavy smoker | Current daily smoker (≥20 cigarettes/day) |
| Light smoker | Current daily smoker (<20 cigarettes/day) or current occasional smoker with ≥100 lifetime cigarettes | |
| Former heavy smoker | Former daily smoker (≥20 cigarettes/day) | |
| Former light smoker | Former daily smoker (<20 cigarettes/day) or former occasional smoker with ≥100 lifetime cigarettes | |
| Non-smoker | Never smoker or occasional smoker <100 lifetime cigarettes | |
| Alcohol | Heavy drinker | Bingeing‡ or >21 (men) or >14 (women) drinks/week |
| Moderate drinker | ≤21 (men) or ≤14 (women) drinks/week with no bingeing | |
| Non-drinker | No alcohol consumption in the last 12 months | |
| Diet | Poor diet | Index score† 0 to <2.5 |
| Fair diet | Index score 2.5 to <5 | |
| Adequate diet | Index score 5 to 10 | |
| Physical activity | Inactive | 0 to <1.5 METs/day |
| Moderately active | 1.5 to <3 METs/day | |
| Active | ≥3 METs/day |
*Highest risk levels are in bold and lowest risk levels (reference group) are in italics.
‡Bingeing: five or more drinks on any day in the previous week or weekly bingeing behaviour in the previous month.
†Index score: the healthiness of a diet based on consumption of fruit and vegetables. Individuals start with 2 points and achieve up to 8 additional points for each average daily serving of fruits and vegetables (maximum score = 10). Points are deducted for daily fruit juice servings exceeding 1 (-2 points), no carrot consumption (-2 points), or daily potato consumption exceeding 1 serving for males and 0.7 servings for females (-2 points). Scores that result in negative values after deductions are recoded to zero, resulting in a final range of 0 to 10 for the index.
MET, metabolic equivalent of task (a measure of calories burned by type, duration and frequency of physical activity).
We examined person-level health care costs across three sectors: 1) hospital care (inpatient hospitalizations, same day surgeries, emergency department visits, rehabilitation hospitals, and complex continuing care centres); 2) drugs (for Ontarians age 65 and older and Ontarians receiving social assistance, Ontario Drug Benefit costs were captured); and 3) community care (primary care billings, specialist billings, lab billings, capitation services, and home care services).
Costs to operate the health care system (e.g., health ministry administrative costs) and capital costs for large scale projects (e.g., building new hospitals) are not reflected in the person-level costs. To account for these exclusions in the health care cost analysis, we obtained annual total health care expenditures from publicly available Ontario Ministry of Finance records (fiscal years 2003 to 2013, where fiscal year is April 1 to March 31), the Canadian Institute for Heath Information’s National Health Expenditure Trends publication, and the MOHLTC’s Report Card for the Ontario Drug Benefit Program publications11–23. The expenditures from each year were categorized into our three health care sectors and expenditures that did not correspond with any of the three sectors were assigned to an ‘other’ category.
All costs are expressed in 2014 Canadian dollars with past costs inflated using the annual general Consumer Price Index reported by Statistics Canada.
To develop multivariable cost models, Ontario respondents to the 2003, 2005 and 2007-2008 cycles of CCHS were linked, at the individual level, to all records of health care use that were paid for by the Ontario Ministry of Health and Long-Term Care (MOHLTC). The cost associated with each record was estimated using costing methods developed for Ontario health administrative data24. Briefly, a payer (the MOHLTC) cost perspective was taken, using person-level health care utilization and per-use fee information or budgetary data. Cost information for sectors (i.e., acute hospitalization, same day surgery, emergency department, inpatient rehabilitation, and complex continuing care) that are funded using global budgets (e.g., by institution) are determined using a top-down approach through case-mix methodology. Sectors that have fee payments associated with each use (e.g., prescription drugs, physician services, and home care) have costs estimated directly.
Beginning one year after survey administration, CCHS respondents were followed for a four-year period (between 2004 and 2013) to develop multivariable models estimating the effect of health behaviours on health care costs. We used a generalized linear model with a negative binomial distribution and an offset to account for variation in follow-up times to create separate, sex-specific models for each of the health care sectors: hospital care, drugs, and community care. To assess confounding and mediation, we use a pre-specified, stepwise modelling approach. We started with a health behaviour model followed by a basic sociodemographic model, a primary attribution model that adjusted for additional sociodemographic risk factors, a distal mediator model that included health status indicators, and a proximal mediator model that included a measure of fragility (see Figure 2). This stepwise analysis resulted in 15 models for each sex.
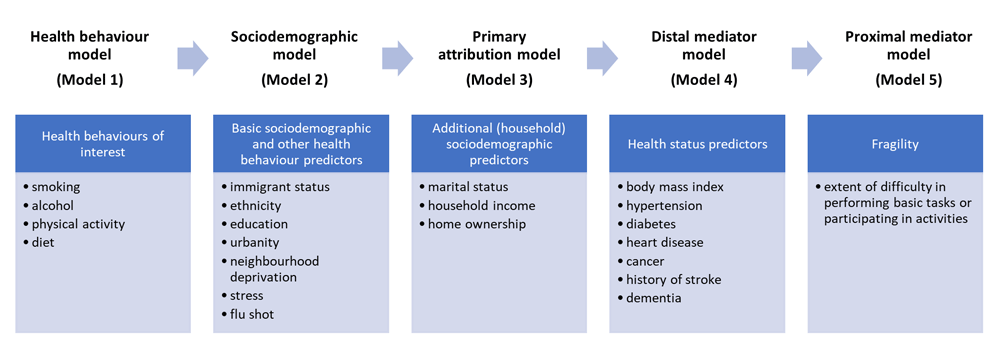
The model building approach sought to address three issues in assessing the contribution of health behaviours to health care costs. First, we were interested in having appropriate adjustment for other risk factors for increased costs that are correlated with health behaviours (e.g., age and sociodemographic risk factors). Second, we were attentive to risk factors that may mediate the relationship between health behaviours and health care costs (e.g., body mass index or hypertension). Our concern was that their inclusion in the model could inappropriately attenuate the risk from health behaviours. Third, we considered pre-existing illness that may have led to health behaviour change. For example, as people become ill and frail they may become less physically active; in such a situation, physical inactivity may be associated with increased health care cost that is more appropriately identified as illness-associated inactivity.
We calculated the proportion of the health care costs that can be attributed to health behaviours—the population attributable fraction—for fiscal years 2003 to 2013, for each health care sector. Using the unlinked CCHS cohort, we estimated annual population attributable fractions using each CCHS cycle and for years between CCHS cycles, by averaging the population attributable fractions from the preceding and succeeding year.
We used a factor-deleted approach to calculate population attributable fractions that involved three steps. In the first step, we estimated expected annual population health care costs for a specific sector by applying the corresponding sector-specific primary attribution models to the weighted CCHS cycle. In the second step, we repeated the calculation after recoding each respondent’s health behaviour to the counterfactual reference or “no exposure” category. For example, taking the weighted cohort, we estimated hospital costs using all smoking exposures (i.e., current, former, and non-smokers) and re-estimated hospital costs assuming all current and former smokers were non-smokers. The difference between the two calculations was an estimate of the annual contribution of smoking to hospital costs. In the final step, we divided this difference by the original population estimate (from the first step) to produce a population attributable fraction. In our example, this would be the population attributable fraction of hospital costs associated with smoking. Health sector specific population attributable fractions were calculated for each health behaviour, and the combination of health behaviours. The same analysis was performed for different socioeconomic groups defined by education, family income, home ownership, and neighbourhood deprivation.
We calculated annual estimates of costs attributable to health behaviours and socioeconomic position (fiscal years 2004 to 2013) by applying the sector-specific population attributable fractions to the annual public health care expenditures and summing the health care sector results together.
The change in health care costs attributable to the change in health behaviours was estimated for fiscal years 2004 to 2013. A baseline population attributable fraction for total health care costs associated with all health behaviours was estimated for fiscal year 2003 using the previously described methods for population attributable fractions and attributable costs. The overall health care budget was estimated over the subsequent decade, assuming that health behaviours in 2003 remained constant (e.g., the baseline population attributable fraction did not change over time). The difference between the counterfactual health care budget and the actual health care budget provided an annual estimate of the change in health care costs attributable to changes in health behaviours.
We performed three sets of sensitivity analyses. First, we created five models representing a stepwise approach to assess confounding and mediation. The five models assessed health care costs and burden estimates with progressive adjustment for risk factors: age, sex and health behaviours (Model 1), basic socio-demographic characteristics (Model 2), additional sociodemographic characteristics (Model 3), health status mediators (Model 4), and fragility measures (Model 5) (Figure 2). The estimate derived from Model 3 was assumed to be our most accurate and appropriate estimate of the attributable burden due to health behaviours. The estimates derived from Model 1 (simply age and health behaviours) and Model 5 (the over-adjusted model) were used as upper and lower bounds of uncertainty.
For our second sensitivity analysis, we compared age-standardized cost ratios after excluding the top 5% of health care users to assess the possibility of overly influential respondents. The use of health care varies considerably between people, particularly for hospital care and other speciality services. Only a small proportion of people are hospitalized and, of those hospitalized, a small proportion has multiple admissions and complicated long hospital stays. The skewed distribution of health care services has potential to distort the attributable health care expenditure analysis because a small proportion of CCHS respondents may have a strong influence on overall or total population estimates.
Third, we replicated analyses using an inverse propensity-weighted model to assess robustness of the health care cost ratios attributable to smoking. The inverse propensity-weighted model, a complimentary approach to the generalized linear model, is an alternative approach to adjust cost ratios for multivariable risk factors. Our inverse propensity-weighted analyses included several covariates in addition to all those used in the multivariable analyses (i.e., the primary attribution model): household type, highest level of household education, main source of household income, labour force participation, sense of belonging to the community, regional health authority, and survey cycle.
The population attributable fractions for the four behavioural risks were calculated using responses from 80,749 Ontarians surveyed between 2003 and 2008. In total, there were 312,952 person-years of follow-up. Characteristics of the study cohort are presented in Table 2.
| Male cohort | Female cohort | |||||
|---|---|---|---|---|---|---|
| Survey sample* % | Person- years | Represented population‡ % | Survey sample* % | Person- years | Represented population‡ % | |
| (N=36,807) | (N=3,962,088) | (N=43,942) | (N=4,131,570) | |||
| Age group (years) | ||||||
| 25 to 29 | 7.4 | 10,668 | 9.1 | 7.3 | 12,642 | 8.8 |
| 30 to 34 | 9.0 | 13,001 | 10.0 | 8.5 | 14,714 | 9.1 |
| 35 to 39 | 10.3 | 14,957 | 11.5 | 9.1 | 15,888 | 11.5 |
| 40 to 44 | 11.1 | 16,183 | 14.5 | 8.8 | 15,326 | 12.5 |
| 45 to 49 | 9.0 | 13,059 | 11.5 | 8.1 | 14,149 | 11.6 |
| 50 to 54 | 9.6 | 13,847 | 10.2 | 9.4 | 16,256 | 10.2 |
| 55 to 59 | 10.0 | 14,420 | 9.6 | 10.3 | 17,760 | 9.1 |
| 60 to 64 | 9.0 | 12,897 | 7.2 | 9.0 | 15,451 | 7.2 |
| 65 to 69 | 7.9 | 11,152 | 5.6 | 8.0 | 13,568 | 6.2 |
| 70 to 74 | 6.8 | 9,408 | 4.6 | 7.5 | 12,650 | 5.0 |
| 75 to 79 | 5.3 | 7,004 | 3.4 | 6.5 | 10,654 | 4.2 |
| 80 to 84 | 3.1 | 3,907 | 2.0 | 4.8 | 7,575 | 2.8 |
| 85 to 89 | 1.2 | 1,356 | 0.7 | 2.1 | 3,257 | 1.3 |
| 90+ | 0.3 | 320 | 0.2 | 0.7 | 883 | 0.4 |
| Health Behaviours | ||||||
| Smoking status | ||||||
| Heavy smoker | 10.7 | 15,116 | 9.2 | 5.8 | 9,828 | 4.8 |
| Light smoker | 14.5 | 20,642 | 15.4 | 14.8 | 25,234 | 13.8 |
| Former heavy smoker | 20.0 | 27,998 | 16.0 | 9.5 | 16,010 | 7.7 |
| Former light smoker | 17.4 | 24,531 | 16.8 | 18.0 | 30,678 | 16.3 |
| Non-smoker | 36.5 | 52,432 | 41.8 | 51.0 | 87,361 | 56.6 |
| Missing | 1.1 | 1,460 | 0.9 | 1.0 | 1,661 | 0.8 |
| Alcohol consumption | ||||||
| Heavy drinker | 12.3 | 17,695 | 10.9 | 3.4 | 5,762 | 3.3 |
| Moderate drinker | 70.7 | 100,787 | 71.8 | 72.2 | 124,162 | 70.0 |
| Non-drinker | 15.1 | 20,940 | 15.3 | 23.3 | 38,974 | 25.6 |
| Missing | 2.0 | 2,758 | 2.0 | 1.1 | 1,876 | 1.1 |
| Physical activity | ||||||
| Inactive | 46.5 | 65,794 | 47.8 | 52.8 | 89,455 | 54.3 |
| Moderately active | 25.2 | 36,032 | 24.3 | 25.6 | 44,103 | 24.5 |
| Active | 26.1 | 37,473 | 25.1 | 20.8 | 35,913 | 19.7 |
| Missing | 2.2 | 2,880 | 2.8 | 0.9 | 1,302 | 1.5 |
| Diet | ||||||
| Poor diet | 15.0 | 21,400 | 14.2 | 8.6 | 14,632 | 8.3 |
| Fair diet | 41.7 | 59,340 | 40.3 | 30.0 | 50,933 | 29.2 |
| Adequate diet | 38.6 | 55,167 | 40.7 | 58.2 | 99,981 | 59.0 |
| Missing | 4.7 | 6,272 | 4.8 | 3.3 | 5,227 | 3.6 |
| Sociodemographic Indicators | ||||||
| Immigrant status | ||||||
| Immigrant | 21.8 | 30,837 | 33.5 | 21.5 | 36,779 | 33.9 |
| Non-immigrant | 78.1 | 111,177 | 66.2 | 78.3 | 133,765 | 65.8 |
| Missing | 0.1 | 165 | 0.3 | 0.1 | 228 | 0.4 |
| Ethnicity | ||||||
| White | 88.9 | 126,274 | 78.9 | 89.7 | 152,990 | 79.3 |
| Non-white | 10.7 | 15,357 | 20.5 | 10.0 | 17,183 | 20.1 |
| Missing | 0.4 | 548 | 0.6 | 0.4 | 600 | 0.5 |
| Education | ||||||
| Less than high school | 18.9 | 25,984 | 14.8 | 20.2 | 33,702 | 16.5 |
| High school graduate | 22.7 | 32,530 | 22.2 | 25.0 | 42,730 | 24.9 |
| Post-secondary graduate | 57.5 | 82,404 | 61.8 | 54.1 | 93,212 | 57.7 |
| Missing | 0.9 | 1,262 | 1.2 | 0.7 | 1,129 | 0.9 |
| Marital status | ||||||
| Married/common-law | 67.8 | 96,810 | 76.8 | 57.7 | 99,508 | 68.8 |
| Other | 32.2 | 45,306 | 23.2 | 42.3 | 71,197 | 31.2 |
| Missing | 0.0 | 64 | 0.0 | 0.0 | 68 | 0.0 |
| Residence ownership | ||||||
| Yes | 79.3 | 113,229 | 79.9 | 75.9 | 130,283 | 77.8 |
| No | 20.5 | 28,730 | 19.7 | 23.9 | 40,246 | 21.9 |
| Missing | 0.2 | 221 | 0.3 | 0.1 | 244 | 0.3 |
| Household income ($) | ||||||
| 0 to 29,999 | 16.6 | 22,903 | 11.0 | 25.9 | 43,299 | 16.4 |
| 30,000 to 79,999 | 45.5 | 64,687 | 40.1 | 41.6 | 71,561 | 39.3 |
| 80,000+ | 32.2 | 46,643 | 40.3 | 23.5 | 40,774 | 31.0 |
| Missing | 5.7 | 7,946 | 8.6 | 9.0 | 15,140 | 13.3 |
| Preventive healthcare | ||||||
| Flu shot | ||||||
| Yes | 61.6 | 87,077 | 58.0 | 68.6 | 116,714 | 63.4 |
| No | 36.0 | 51,907 | 38.9 | 30.5 | 52,701 | 35.0 |
| Missing | 2.5 | 3,195 | 3.1 | 0.9 | 1,358 | 1.5 |
| Geography | ||||||
| Urban | ||||||
| No | 22.3 | 31,709 | 14.9 | 21.0 | 36,089 | 14.3 |
| Yes | 77.7 | 110,471 | 85.1 | 79.0 | 134,684 | 85.7 |
|
Neighbourhood deprivation | ||||||
| High | 15.1 | 21,394 | 11.8 | 16.3 | 27,519 | 12.6 |
| Moderate | 62.3 | 88,446 | 60.8 | 62.3 | 106,413 | 61.2 |
| Low | 20.6 | 29,532 | 25.3 | 19.3 | 33,198 | 24.2 |
| Missing | 2.0 | 2,807 | 2.1 | 2.2 | 3,643 | 2.0 |
| General health indicators | ||||||
| Self-perceived stress | ||||||
| Quite a bit or extremely stressful | 20.2 | 28,881 | 23.0 | 21.5 | 36,892 | 24.4 |
| At most, a bit stressful | 79.5 | 112,917 | 76.7 | 78.2 | 133,339 | 75.4 |
| Missing | 0.3 | 382 | 0.3 | 0.3 | 542 | 0.3 |
| Body mass index | ||||||
| Underweight | 0.7 | 926 | 0.8 | 2.7 | 4,428 | 3.2 |
| Normal | 43.5 | 62,221 | 43.3 | 29.9 | 51,187 | 28.3 |
| Overweight | 15.6 | 22,224 | 13.9 | 12.5 | 21,474 | 10.9 |
| Obese | 4.8 | 6,884 | 4.0 | 6.3 | 10,740 | 5.3 |
| Morbidly Obese | 34.2 | 48,428 | 36.5 | 45.5 | 77,566 | 48.5 |
| Missing | 1.2 | 1,497 | 1.5 | 3.2 | 5,378 | 3.9 |
| Indicators of illness | ||||||
| Hypertension | ||||||
| Yes | 22.7 | 31,629 | 18.9 | 25.7 | 43,146 | 20.5 |
| No | 77.0 | 110,133 | 80.8 | 74.2 | 127,408 | 79.4 |
| Missing | 0.3 | 417 | 0.3 | 0.1 | 218 | 0.1 |
| Diabetes | ||||||
| Yes | 8.7 | 11,757 | 7.2 | 7.5 | 12,319 | 6.2 |
| No | 91.3 | 130,305 | 92.7 | 92.5 | 158,364 | 93.8 |
| Missing | 0.1 | 118 | 0.1 | 0.1 | 90 | 0.0 |
| Heart disease | ||||||
| Yes | 9.4 | 12,559 | 6.8 | 7.9 | 12,819 | 5.5 |
| No | 90.4 | 129,348 | 93.0 | 91.9 | 157,654 | 94.3 |
| Missing | 0.2 | 272 | 0.1 | 0.2 | 299 | 0.2 |
| Cancer | ||||||
| Yes | 2.9 | 3,754 | 2.1 | 2.6 | 4,199 | 2.0 |
| No | 97.0 | 138,284 | 97.9 | 97.3 | 166,374 | 97.9 |
| Missing | 0.1 | 141 | 0.1 | 0.1 | 200 | 0.1 |
| Stroke | ||||||
| Yes | 2.0 | 2,552 | 1.4 | 1.8 | 2,796 | 1.4 |
| No | 98.0 | 139,540 | 98.6 | 98.1 | 167,826 | 98.6 |
| Missing | 0.1 | 88 | 0.1 | 0.1 | 151 | 0.0 |
| Dementia | ||||||
| Yes | 0.6 | 645 | 0.5 | 0.4 | 629 | 0.5 |
| No | 99.4 | 141,440 | 99.5 | 99.5 | 170,034 | 99.4 |
| Missing | 0.1 | 94 | 0.1 | 0.1 | 109 | 0.1 |
| Fragility | ||||||
| Help with basic tasks | 6.6 | 8,570 | 5.4 | 13.6 | 22,096 | 11.4 |
| Limitation due to health | 21.7 | 30,749 | 18.8 | 20.3 | 34,852 | 17.8 |
| No limitations | 71.4 | 102,527 | 75.5 | 65.8 | 113,449 | 70.6 |
| Missing | 0.3 | 334 | 0.3 | 0.2 | 376 | 0.2 |
From fiscal years 2004 to 2013, 22% of Ontario’s health care costs could be attributed to the four health behaviour risk factors (Figure 3). Physical activity had the largest attribution (13%), followed by smoking (10%). However, uncertainty for the burden estimates indicates potential overestimation for physical activity and underestimation for diet. Alcohol-attributable health care costs were also likely underestimated (see limitations section).
During the 10-year period (2004 to 2013), $89.3 billion in health care costs were attributable to health behaviours. In that same period, the costs attributable to health behaviours improved by 1.9% (23.3% of total healthcare costs in 2004 to 21.4% in 2013). If the proportion of health care expenditure that can be attributed to health behaviours (the population attributable fraction) had remained at 23.3%, use of health care would have been $5.0 billion greater (what we term ‘avoided cost’).
Table 3 presents the burden of health behaviours related to health care costs ($89.3 billion) and the costs avoided by the adoption of healthy behaviours ($5.0 billion). Physical inactivity and smoking contributed the largest proportion of the burden (53% and 41%, respectively). However, a decline in smoking between 2004 and 2013 was responsible for 84% of the avoided costs.
| Risk | Attributable costs ($89.3 billion) | Avoided costs ($5.0 billion) |
|---|---|---|
| Smoking | 41% | 84% |
| Alcohol | 1% | 0% |
| Diet | 5% | 5% |
| Physical activity | 53% | 11% |
The scenarios from our sensitivity analysis demonstrate results that were similar to our main analysis. Not unexpectedly, the attribution of health care costs to health behaviours decreased as we increased the number of risk factors adjusted for in the model. Excluding high-cost health care users demonstrated slightly attenuated age-standardised cost ratios for men and women for almost all health behaviour risks. The inverse propensity-weighted model, which adjusted for additional variables, had similar cost ratios to the main analysis from the multivariable model.
Between 2004 and 2013, $60.7 billion dollars in health care costs (15% of all health care costs for Ontarians aged 25 years and older) were attributable to low socioeconomic position (Figure 4). When health behaviours and socioeconomic position are considered jointly, the health care cost burden was $134 billion (34% of all health care costs for those aged 25 years and older). The break down by health care sector was similar for health behaviour and socioeconomic position. The largest portion of the burden is due to hospital care costs (46% of health behaviour attribution and 54% of socioeconomic position attribution to health care costs); followed by community care costs (22% and 19%), ‘other’ health care costs (21% and 19%), and drug costs (10% and 9%).
We estimated that smoking, unhealthy alcohol consumption, poor diet and physical inactivity attributed to 22% of Ontario’s direct health care costs during the ten-year period from 2004 to 2013. During this same period, improving health behaviours equated to a nearly 2% reduction in direct health care expenditure. Ontarians in the most disadvantaged socioeconomic group contributed to 15% of the province’s direct health care costs. Taken together, health behaviours and socioeconomic position contributed to a burden of $134 billion in direct health care costs (Ontario, 2004 to 2013).
Estimates of the cost of modifiable health behaviours and socioeconomic risk factors provides evidence to allow policy-makers to prioritize interventions aimed at reducing health care costs. Our estimate of a 1.9% reduction in health care expenditure (i.e., $5 billion) through improved health behaviours, suggests that investments in promotion of healthy living have potential for substantial savings in health care costs in Canada. In our study, reduced smoking was the main contributor to avoided health behaviour attributable health care costs (accounting for 84% of the 1.9% cost reduction). This large cost reduction reflects prominent smoking prevention strategies that were introduced during the study period—including 100% smoke-free public places including restaurants25. From 2004 to 2013 there was an 11% reduction in the attributable fraction of smoking, which was solely a consequence of a reduction in the prevalence of current smoking. The large remaining burden from health behaviours and social inequalities suggests that there are significant opportunities to further reduce health care costs through population health strategies.
It is difficult to compare our findings with previous studies for two main reasons. First, various studies have estimated the economic burden in terms of costs for treatment and management of chronic diseases related to smoking, alcohol, diet or physical activity, but very few have evaluated the simultaneous impact of multiple risk factors in a population. These latter studies have used traditional population aggregated-data attributable fraction methods to estimate economic burden26–31. Second, the significant methodological differences between our study and previous literature limits direct comparison of findings. Briefly, traditional population attributable fraction methods identify diseases where health behaviours are risk factors, estimate the health care costs of these diseases, calculate the proportion of the disease that can be attributed to the risk factors (based on relative risk of disease from an external source and prevalence of exposure in the population of interest), and apply these population attributable fractions to the cost data. There are limitations associated with these methods that stem from combining ecological summary measures of exposure, outcome, and hazards across different sources of data32. Our use of multivariable algorithms and the direct attribution of health behaviours to health care costs offers advantages over analyses that have been performed to date: controlling for confounders, accounting for complexities in the relationship between multiple exposures and covariates, using consistent definitions of exposure, and using specific measures of risk derived internally from the study population.
Our study has several limitations that we believe underestimate the actual burden of health care costs attributable to the four health behaviours. First, we excluded individuals younger than 25 years of age. In general, health care costs for this age group are small; however, alcohol burden for younger people is a notable omission. Alcohol has an important attribution for injury, suicide and other social burdens that occur disproportionately among young people33–35. Second, our reliance on self-reported health risk exposures will generally result in an underestimation of risk burden, especially for food and alcohol36–39. In what is referred to as “social desirability bias”, survey respondents tend to over-report what they perceive as healthy behaviour and underreport unhealthy behaviour40. As an example, self-reported alcohol consumption in surveys accounts about half the volume of alcohol sold41–42. While reporting accuracy affects all risks in this study, burden estimates are mostly affected when people report they are in the healthiest category and they are actually in an unhealthy category. Third, the survey’s brief questions about risks may not capture the full spectrum of behaviour. For example, our measure of physical activity (leisure-time physical activity) did not include active transportation (such as walking and bicycling to work), work activity, or sedentary time and our measure of diet (fruit and vegetable consumption) did not specifically ascertain intake of sodium, trans fats, calories or other aspects of healthy and unhealthy eating. Fourth, we used respondents’ answers to health behaviours and other risks that correspond to their health behaviour at the time of the survey. In general, studies that consider lifetime changes in risks generate higher burden estimates. Our physical activity burden estimates may be an exception, where reverse-causality (i.e., ill health is the cause of reduced physical activity) results in overestimation. Fifth, “other” health care costs—including health care system operating costs and capital costs—are not included our health care cost data and were estimated indirectly. Sixth, we did not include indirect costs (such as lost productivity, wages and income related to illness associated with unhealthy living, or costs borne by individuals to care for their illness), nor did we include costs for health care beyond those paid by the provincial government (e.g., employee health plans).
Despite these limitations, our study demonstrates the importance of integrating public health and prevention within the health care system. A greater investment in disease prevention and population health could help increase the sustainability of publicly funded health care by reducing spending on illness, particularly with respect to hospital care. Combining this effort with strategies that address social determinants of health could secure further benefits. Indeed, our study suggests that interventions outside the health care system, such as improving levels of income and education, and other risk factors that influence socioeconomic position, will reduce health care costs. The health system is one determinant of population health and attention must be paid to the needs of disadvantaged individuals, populations, and communities in order to avoid increasing health disparities43. While health inequities play a significant role in health system costs, estimating the cost of the gap in health care as a result of socioeconomic position can be difficult because of the complexity of the problem. Our study is one of the few that has been able to translate this gap into a dollar figure.
Our study shows that health behaviours and socioeconomic position contribute to a large health care cost burden. Better health and well-being is the primary goal of improving health behaviours and reducing social inequity. However, it is important to recognize that existing investments in public health also results in a large reduction in expenditure in the acute care sector, particularly hospital care. The health premium of prevention and social equity is an overlooked opportunity for a sustainable health care system.
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Open Science Framework: Burden of health behaviours and socioeconomic position on health care expenditure in Ontario. https://doi.org/10.17605/OSF.IO/KW4C544.
This project contains the sensitivity analysis supplementary file.
Extended data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful for the contributions of Sima Gandhi and Erika Yates as well as our Scientific and Policy Advisory Committees—namely, Peter Austin, Bernard Choi, Prabhat Jha, Isra Levy, Heather Manson, Claudia Sanmartin, Joanne Thanos, and Pegeen Walsh.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Ding D, Kolbe-Alexander T, Nguyen B, Katzmarzyk PT, et al.: The economic burden of physical inactivity: a systematic review and critical appraisal.Br J Sports Med. 2017; 51 (19): 1392-1409 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Lifestyle epidemiology, physical activity and health, cost of illness analysis.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology of chronic diseases, cardiovascular risk prediction modelling, linked administrative health data
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 Oct 19 |
read | |
|
Version 1 18 Mar 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)