Keywords
IL-17, psoriasis, Acrodermatitis continua of Hallopeau, paradoxical reaction
IL-17, psoriasis, Acrodermatitis continua of Hallopeau, paradoxical reaction
Psoriasis is an immune-mediated inflammatory disease characterized by a chronic course and a systemic involvement. Numerous cytokines are involved in the pathogenesis of psoriasis, but the interleukin (IL)-23/17 axis has been identified as one of the key pathways1. Up to 40% of patients with psoriasis may develop psoriatic arthritis (PsA). Both conditions share common pathogenic mechanisms2. Among the therapies that can be used for both skin and joint manifestations, anti-TNF agents and new biologics targeting IL-17 have shown impressive efficacy3. We report the case of a patient who presented with an acrodermatitis continua of Hallopeau (ACH)-like paradoxical reaction to anti-IL17 therapy for PsA.
A 52-year-old-woman affected by PsA presented to our Dermatology Unit complaining of a painful eruption of pustules with scaling and tender swelling on the fingers of both hands (Figure 1), which had begun one month before. Her medical history revealed concurrent PsA in complete remission after 10 months of the, the anti-IL-17 drug, secukinumab, at the dosage of 150mg every 4 weeks. The patient did not suffer from any other relevant disease and there was no family history of psoriasis.
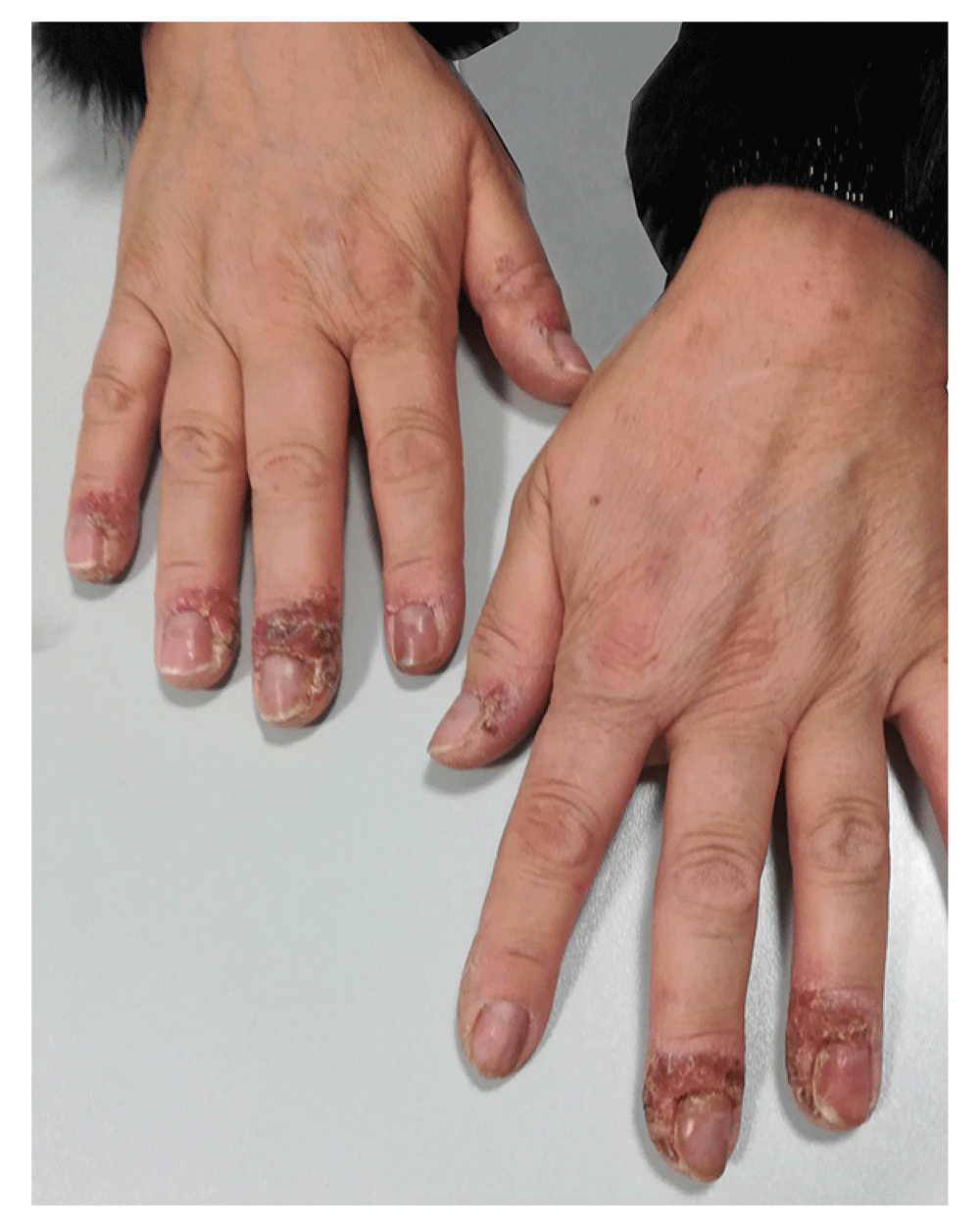
A skin biopsy was taken, and the subsequent histopathological examination showed a stratified squamous epithelium with parakeratosis, hyperkeratosis and irregular elongation of the rete ridges of the epidermis with some lymphocytes and subcorneal collections of neutrophils forming spongiform pustules of Kogoj. This result, together with clinical features and negative results of multiple cultures confirmed our suspect of an ACH-like eruption4.
It was then decided to stop anti-IL-17 therapy. A subsequent treatment plan comprised topical clobetasol propionate, once daily, and acitretin 10mg daily.
At the follow-up visit 2 months later, the lesions had visibly regressed and the patient referred a 70% reduction in symptoms measured with Dermatology Life Quality Index questionnaire. The interruption of secukinumab notwithstanding, PsA showed no recrudescence.
The natural history of psoriasis has been modified in the last years by new biologic agents that have allowed specific targeting of key cytokines such as TNF alpha, IL-12, IL-23 and IL-17.
TNF alpha inhibitors are the oldest, and therefore most studied, biologic drugs that have been introduced in the therapy of psoriasis. By inhibiting the whole pathway, TNF alpha blockers determine a stronger alteration of cytokine network5. This has been associated not only with impressive efficacy, but also remarkable side effects, such as infections, autoimmune diseases, lymphomas and cutaneous adverse events, mainly represented by paradoxical psoriasis6.
In the last years, new biologic therapies targeting cytokines situated downstream to TNF alpha, such as ustekinumab, secukinumab and, more recently, ixekizumab have shown even higher efficacy due to their selectivity in blocking cytokines specific to the pathogenic pathways. Also for this drug, although uncommon, important adverse reactions such as opportunistic infections, can be observed7.
In the literature, one case of paradoxical psoriasic reaction has recently been described in association to secukinumab8. In our case report we have observed an unexpected case of paradoxical ACH-like eruption, which to the best of our knowledge, has not been described in literature as an adverse event of secukinumab.
It is possible that secukinumab, by blocking IL-17, has induced a rearrangement of the cytokine pattern in this patient, determining a paradoxical increase of other pathogenic molecules, such as TNF alpha. This hypothesis has already been suggested for paradoxical reactions to ustekinumab, which exerts a blockade of IL12 and IL239. On the other hand, is also possible that the inhibition of IL17 induced a negative feedback in the IL23-IL17 axis, thus determining an increase of IL23 which, in turn, stimulated Th17 cells to produce other cytokines, such as IL-22, which also exerts proliferation and activation of keratinocytes. Activated keratinocytes could induce the chemotaxis of neutrophils (IL-8 etc), causing the clinical presentation that we have observed in our patient10. This is the first case report describing ACH-like lesions induced by secukinumab; therefore few studies are available in the literature elucidating the pathogenesis of this paradoxical reaction. Hence, our experience needs to be reinforced by further investigations.
It is likely that our patient was genetically prone to respond exaggeratedly to a cytokine imbalance. The distinction of such patients could be useful in the future, in order to predict this type of adverse reaction and, therefore, suggesting them an alternative to biologic therapies.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Sladden M, Sladden C, Gulliver W: Secukinumab-Induced Psoriasiform Eruption. JAMA Dermatology. 2017; 153 (11). Publisher Full TextCompeting Interests: I have been an investigator, speaker, advisory board member, and consultant for Novartis.
Reviewer Expertise: Psoriatic disease, clinical trials, real world experience
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology of infected diseases, Immune response to tuberculosis and influenza, TLR signaling, inflammation, host directed therapy and vaccine mediated immunity.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Mar 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)