Keywords
Arabidopsis, Pollen tube, fertilization recovery system, pollen tube-dependent ovule enlargement morphology, GCS1, pollen tube contents, Seed coat initiation, AGL62
Arabidopsis, Pollen tube, fertilization recovery system, pollen tube-dependent ovule enlargement morphology, GCS1, pollen tube contents, Seed coat initiation, AGL62
We have changed the figure 1E. Blue bars indicate SF (Seed formation ratio). Dark red bars indicate VSW (vanillin stained in whole seed coat). Gray bar indicates SA (Seed abortion ratio). Pink bars indicate VSP (vanillin stained in partial seed coat).
See the authors' detailed response to the review by Nobutaka Mitsuda
See the authors' detailed response to the review by Tomoko Igawa
See the authors' detailed response to the review by Tomokazu Kawashima
In angiosperms, seed formation begins with pollination1,2. Once the pollen grain lands on the stigma at the top of the pistil, pollen tubes from the grain elongate toward the ovule. Fusion of the two gametes is required for seed formation. The male gametophyte is the pollen grain and the female gametophyte is the embryo sac3. Immediately after arrival at the ovules, the pollen tube bursts and the pollen tube contents (PTC) are released to the female gametophyte4.
In a previous study, we reported that once the ovule accepts the PTC inside the female gametophyte, it begins enlargement and seed coat formation, irrespective of fertilization5,6. We named this phenomenon pollen tube-dependent ovule enlargement morphology (POEM). We also reported that if fertilization of the ovule fails, a partial seed coat is still produced, even though a complete seed coat cannot be formed. However, we have not confirmed whether all the ovules have the partial seed coat phenotype when ovule fertilization fails but PTC is accepted. To address this question, statistical experiments were conducted that included the fertilization recovery system (FRS), where a second pollen tube rescues the fertilization if fertilization by the first pollen tube fails, which we previously identified7,8. We reported that the seed formation ratio of gcs1/+ mutants9–11 was approximately 65%; the remaining mutants were unable to produce seeds because fertilization of these ovules failed. Therefore, matching of the ratio of the ovules with the partial seed coat phenotype to the seed abortion ratio suggests that there was fertilization failure for all ovules with a partial seed coat. We also conducted experiments to determine whether agl62 mutants12 had the partial seed coat phenotype. In agl62 seeds, the endosperm cellularizes prematurely, indicating that AGL62 is required for suppression of cellularization during the syncytial phase. During seed development, AGL62 is exclusively expressed in the endosperm. Because agl62 mutants have an abnormal endosperm phenotype after central cell fertilization, these mutants are ideal for investigating the relationship between endosperm formation and seed coat initiation and formation.
Arabidopsis thaliana ecotype Columbia (Col-0) plants were used as the wild-type (WT) plants. Test cross experiments were conducted in gcs1/+9–11, agl62/+12, and WT plants. Seeds were sterilized with 5% sodium hypochlorite containing 0.5% Triton X-100 and germinated on plates containing 0.5× Murashige and Skoog salts (pH 5.7) (Wako Pure Chemical), 2% sucrose, Gamborg’s B5 vitamin solution (Sigma), and 0.3% Gelrite (Wako Pure Chemical) in a growth chamber at 21.5°C under 24 h of light after cold treatment (4°C) for 2 days. Next, 10-day-old seedlings were transferred to Metro-Mix 350 soil (Sun Gro) and grown at 21.5°C under 24 h of light.
For staining the silique tissue, the WT flowers were emasculated at stage 12c13 and pollinated with gcs1/+ pollen grains. For agl62 experiments, the agl62 mutant flowers were emasculated at stage 12c and pollinated with WT, gcs1/+, and agl62 pollen grains. The siliques were collected at 3 days after pollination (DAP).
For vanillin staining, the ovules were manually dissected from the ovaries and mounted on slides in 1% (wt/vol) vanillin (4-hydroxy-3-methoxybenzaldehyde; Sigma) in 6 N HCl solution. Slides were analyzed after 20 min of incubation. Samples were analyzed with a Leica DM2500 microscope using differential interference contrast optics. Images were recorded with a Leica DFC 300 FX digital camera at a magnification of 5×, 10× and 20×. The microscopic protocols followed were as previously described5.
First, the WT plants as both the female and male parent (Figure 1A) were crossed and the silique after vanillin staining at 3DAP was observed. The ratio of full seed coat formation was 98.7±1.2% (mean ± SD; n=10 pistils), which was consistent with our previous WT fertilization data7,8. By contrast, when the WT plants as the female parent and gcs1/+ as the male parent were crossed, the ratio of full seed coat formation was 68.7±5.8% (n=10 pistils) and the ratio of partial seed coat formation was 32.2±6.5% (n=10 pistils), which also was consistent with our previous gcs1/+ fertilization data. These data suggest that all successfully fertilized ovules produce a full seed coat and all unfertilized but PTC accepted ovules produce a partial seed coat.
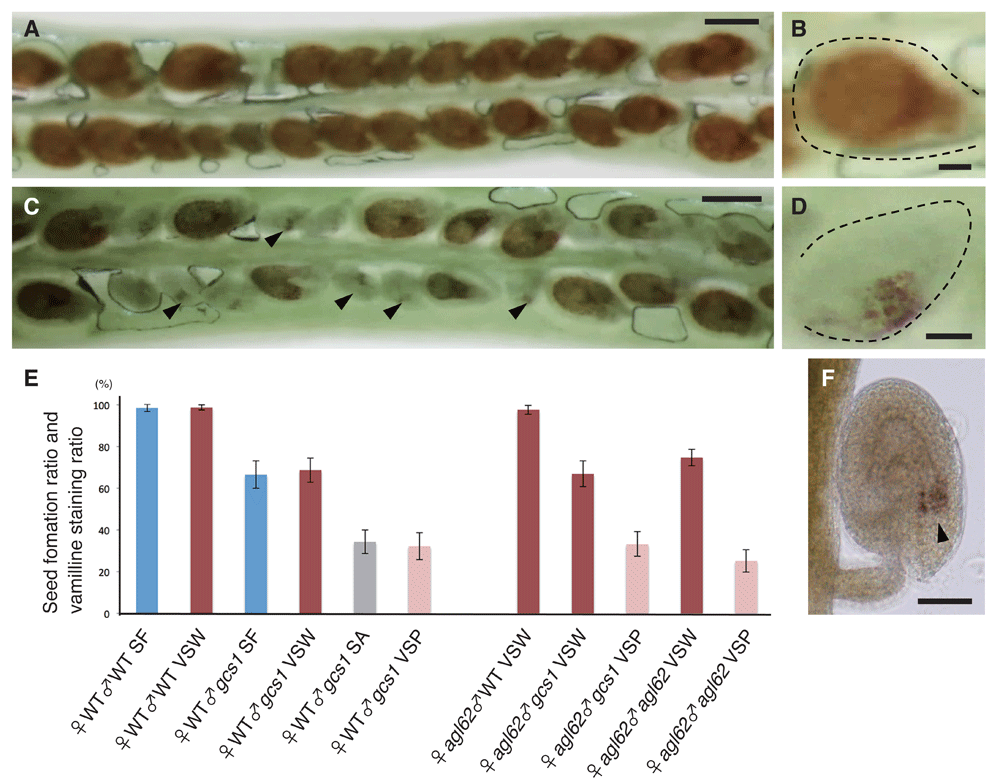
(A) Wild-type (WT) silique crossed with WT pollen and stained with vanillin. Almost all ovules were stained. Bar: 300µm. (B) Representative image of whole seed coat staining. Bar: 50µm. (C) WT silique crossed with gcs1 pollen and stained with vanillin. Several ovules had partial seed coat (arrowhead) staining. Bar: 300µm. (D) Representative image of partial seed coat staining. Bar: 50 µm. (E) Comparison of seed formation ratio and vanillin staining ratio. ♀WT♂WT seed formation ratio (SF) indicates that a WT silique was crossed with WT pollen and the seed formation ratio was calculated. ♀WT♂WT vanillin stained in whole seed coat (VSW) indicates that a WT silique was crossed with WT pollen and the whole seed coat staining ratio was calculated. ♀agl62♂WT VSW indicates that an agl62/+ silique was crossed with WT pollen and the whole seed coat staining ratio was calculated. ♀WT♂gcs1 SF indicates that a WT silique was crossed with gcs1/+ pollen and the seed formation ratio was calculated. ♀WT♂gcs1 VSW indicates that a WT silique was crossed with gcs1/+ pollen and the whole seed coat staining ratio was calculated. ♀agl62♂gcs1 VSW indicates that an agl62/+ silique was crossed with gcs1/+ pollen and the whole seed coat staining ratio was calculated. ♀WT♂gcs1 seed abortion ratio (SA) indicates that a WT silique was crossed by gcs1/+ pollen and the seed abortion ratio was calculated. ♀WT♂gcs1 (vanillin stained in partial seed coat) indicates that a WT silique was crossed with gcs1/+ pollen and the partial seed coat staining ratio was calculated. ♀agl62♂gcs1 VSP indicates that an agl62/+ silique was crossed with gcs1/+ pollen and the partial seed coat staining ratio was calculated. ♀agl62♂ agl62 VSW indicates that an agl62/+ silique was crossed with agl62/+ pollen and the whole seed coat staining ratio was calculated. ♀agl62♂agl62 VSP indicates that an agl62/+ silique was crossed with agl62/+ pollen and the partial seed coat staining ratio was calculated. Blue bars indicate SF (Seed formation ratio). Dark red bars indicate VSW (vanillin stained in whole seed coat). Gray bar indicates SA (Seed abortion ratio). Pink bars indicate VSP (vanillin stained in partial seed coat). (F) A ♀agl62♂agl62 VSP ovule. The arrowhead indicates the vanillin-stained zone. Bar: 50 µm. For normal seed coat initiation, fertilization is not required; however, for completion of normal seed coat formation, both normal fertilization and normal endosperm development are required.
Because the agl62 mutant had an abnormal and arrested endosperm formation phenotype after fertilization, this mutant was ideal for investigating the relationship between endosperm formation and seed coat initiation and formation. The agl62/+ plants as the female parent and the WT as the male parent were crossed (Figure 1) and the silique after vanillin staining at 3DAP was observed. The ratio of full seed coat formation was 97.6±2.1% (n=10 pistils), which was consistent with our previous WT fertilization data. By contrast, when agl62/+ plants as the female parent and agl62/+ as the male parent were crossed, the ratio of full seed coat formation was 74.7±3.9% (n=10 pistils) and the ratio of partial seed coat formation was 25.2±5.4% (n=10 pistils), which was consistent with previous agl62 data12. These results suggest that normal endosperm development is required for completion of seed coat formation, irrespective of fertilization. When agl62/+ plants as the female parent and gcs1/+ as the male parent were crossed, the ratio of full seed coat formation was 66.9±6.2% (n=10 pistils) and the ratio of partial seed coat formation was 33.2±5.9% (n=10 pistils), which also was consistent with our previous gcs1/+ fertilization data. These results suggest that agl62/+ abnormal endosperm prevents normal seed coat formation, but these ovules still produce a partial seed coat because these ovules had accepted the PTC. In summary, for normal seed coat initiation, fertilization is not required; however, for completion of normal seed coat formation, both normal fertilization and normal endosperm development are required.
Open Science Framework: Vanillin staining project. https://doi.org/10.17605/OSF.IO/6U73H14.
This project contains the following underlying data:
# of seeds data (Sheet2 contains the number of seeds stained out of the total number of seeds; Sheet1 the data summary used to produce Figure 1E)
agl62-3v.tif (raw image of stained agl62/+ seeds)
gcs1 vaniline.tif (raw image of stained gcs1/+ seeds)
WT vaniline.tif (raw image of stained wild-type seeds)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
This work was supported by start-up funds from the School of Life Sciences, Fujian Agriculture and Forestry University (Grant #: 114-712018008) and the FAFU-UCR Joint Center and Fujian Provincial Key Laboratory of Haixia Applied Plant Systems Biology, Haixia Institute of Science and Technology, Fujian Agriculture and Forestry University.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gamete imaging during double fertilization in flowering plants, cytological and morphological analysis of sexual plant reproduction processes, molecular biology focusing on proteins regulating fertilization
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Kasahara RD, Notaguchi M, Nagahara S, Suzuki T, et al.: Pollen tube contents initiate ovule enlargement and enhance seed coat development without fertilization.Sci Adv. 2016; 2 (10): e1600554 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Sexual plant reproduction, cellular dynamics, molecular biology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: plant biotechnology, transcription factor, reproductive development, cell wall, seed, cuticle
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Kang IH, Steffen JG, Portereiko MF, Lloyd A, et al.: The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis.Plant Cell. 2008; 20 (3): 635-47 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Gamete imaging during doubler fertilization in flowering plants, cytological and morphological analysis of sexual plant reproduction processes, molecular biology focusing on proteins regulating fertilization
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 30 Apr 19 |
read | ||
|
Version 1 28 Mar 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)