Keywords
TAVR, aortic valve, transcatheter, patient preference, benefit-risk analysis
TAVR, aortic valve, transcatheter, patient preference, benefit-risk analysis
The present version of this manuscript updates previous versions to incorporate feedback provided through peer review, including updates to the discussion section to clarify some of the assumptions made, and to include more reflections on the potential challenges associated with the adapted swing weighting methodology.
See the authors' detailed response to the review by Megan Coylewright
See the authors' detailed response to the review by Juan Marcos Gonzalez
See the authors' detailed response to the review by Ross Jaffe
Aortic stenosis (AS) is a progressive cardiovascular condition resulting from narrowing (or stenosis) of the aortic valve. This narrowing prevents the valve from fully opening, decreasing blood flow out of the heart and forcing it to work harder to maintain sufficient blood flow. If left untreated, AS can lead to severe cardiovascular complications and death1. As of 2015, 12.4% of the United States population over age 75 (nearly 2.5 million people) were reportedly diagnosed with AS2,3. More than one in eight people (13.3%) over the age of 75 in the US have moderate-to-severe AS4. Patients with AS may be asymptomatic for many years until the valve is narrowed severely enough to cause symptoms. Symptoms of AS include chest pain and angina, syncope, dyspnea, fatigue, and palpitations, all of which are exacerbated by physical activity5. Undertreatment of AS patients is common, with more than half of patients referred to cardiologists failing to receive surgical treatment6. Once symptoms appear, often between the ages of 70 and 80 years old, the prognosis of untreated patients is poor7. Among untreated patients, average survival is 50% at two years and 20% at five years after the onset of AS symptoms.
The traditionally recommended treatment for AS is surgical aortic valve replacement (SAVR). Such invasive surgery, involving a large incision in the chest, may not be suitable for all patients, especially those with comorbidities. The alternative, transcatheter aortic valve replacement (TAVR), is a minimally invasive, catheter-based procedure to replace the aortic valve in patients with AS. TAVR is the first-line therapy for inoperable patients with severe AS and an alternative to SAVR in operable high-risk patients. Among patients who are at intermediate surgical risk, both TAVR and SAVR are associated with improvements in disease-specific and generic health status8. However, TAVR is associated with a reduced rate of complications and a quicker recovery time, with patients returning to a normal quality of life more quickly9. When first available, the benefits of TAVR were offset by reportedly increased risks of stroke and the need for a pacemaker10–12. Recent clinical data reveal similar, if not improved rates of stroke and need for pacemaker among TAVR patients13. Furthermore, at a median two-year follow-up, all-cause mortality for patients undergoing TAVR was 20.2% compared with 21.9% for patients undergoing SAVR14.
The choice of TAVR or SAVR involves patients making trade-offs between multiple treatment attributes, including invasiveness, speed of recovery, mortality rates, and risks of complications. Given the challenging nature of this decision, tools have been developed to support patient decision-making15. However, little is known about the weights that patients assign to each attributes, how they make trade-offs between these attributes, and whether and how these preferences vary between patients. The objective of this research was to determine which outcomes associated with TAVR and SAVR patients consider most important, collect quantitative data about how patients weigh the benefits and risks associated with TAVR and SAVR, and to use this data to evaluate patients’ preferences for SAVR or TAVR.
Interim results from the first 93 participants enrolled into this study were published on 21 June 2019 as Version 3. This manuscript reports the final results from this study, based on a sample of 219 participants.
Patients’ preferences for TAVR or SAVR were assessed using a quantitative benefit-risk assessment (BRA). This involved identifying attributes that distinguish TAVR and SAVR, measuring TAVR and SAVR performance on these attributes, eliciting patients’ preferences for these attributes, and combining performance and preference in a BRA to determine what proportion of patients prefer TAVR or SAVR. The patient-preference survey upon which the BRA is based was fielded from July 2018 – January 2019.
A long list of potential attributes was identified by reviewing the attributes highlighted in previous patient preference studies for heart valve surgical interventions, published meta-analyses and clinical studies, and regulators’ assessments of related products. Additional attributes were identified based on consultation with TAVR and SAVR clinical experts and from patient input. The final attributes used in the study were selected based on clinical and regulatory relevance, whether or not the attribute distinguishes between TAVR and SAVR, and to comply with the attribute set properties required of an additive BRA16. For example, to avoid overlap with ‘mortality’, the ‘stroke’ attribute was defined as ‘disabling, non-fatal strokes.’
Descriptions of the final attributes included in the BRA are summarized in Table 1.
TAVR and SAVR performance against the final attributes were identified from the published literature and from clinical data (Table 2), focusing on data that had a high degree of reference and use within the clinical community. Available data for stroke risk, defined as “all stroke” (both fatal and non-fatal) in the literature, and independence, defined by Kansas City Cardiomyopathy Questionnaire (KCCQ)17 score, required transformation to estimate performance as defined by the final study attributes. To estimate the risk of non-fatal stroke only, available stroke risk data was adjusted using the mortality rate among patients with severe aortic stenosis enrolled in the PARTNER trial who suffered a stroke compared to the mortality rate among those in the trial who did not suffer a stroke18. ‘Independence’ was defined as the probability of achieving relief from AS symptoms within a month of a procedure. Given available data, this was estimated as the probability of achieving a total score of 75 on KCCQ17. The KCCQ is a standard patient reported outcome measure used in clinical trials of surgical and transcatheter heart valves21. Estimated mean KCCQ score and variation in this measure were transformed into the proportion of patients achieving a KCCQ total score of 75 at 1 month using procedures previously described in Marchini22.
| Attribute | Measure | TAVR | SAVR | Performance Range | ||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||
| Mortality (all cause)19 | One-month risk | 0.011 | 0.005-0.017 | 0.040 | 0.028-0.053 | 0.005-0.053 |
| Disabling non-fatal stroke19 | One-month risk | 0.008 | 0.002-0.013 | 0.033 | 0.021-0.044 | 0.002-0.044 |
| Independence8 | Probability of having relief from AS symptoms that have an effect on daily life | 0.479 | 0.454-0.500 | 0.249 | 0.227-0.276 | 0.227-0.500 |
| New permanent pacemaker19 | One-year risk | 0.123 | 0.103-0.142 | 0.090 | 0.072-0.108 | 0.072-0.142 |
| Requirement for dialysis20 | One-year risk | 0.032 | 0.021-0.042 | 0.047 | 0.034-0.060 | 0.021-0.060 |
| Time over which the Procedure has been Proven to Work | Years | 10 | 20 | 5 - 30 | ||
An adapted swing weighting (ASW) exercise was administered online to elicit patients’ preferences for treatment attributes23. The objective of the ASW exercise was to identify the level of change in an attribute that patients would be willing to accept in exchange for improving their procedure from ‘invasive’ to ‘minimally invasive’ (see Table 1 for definitions). The ASW exercise consisted of a series of pairwise comparisons of attributes—the ‘invasiveness’ attribute and one other attribute. Participants were shown ‘current’ and ‘improved’ levels on each attribute and asked which improvement they would prefer to make (an example choice question is shown in Figure 1). The ‘current’ levels were chosen to reflect the attribute performance levels of TAVR and SAVR (Table 2), to ensure they had credibility with patients, adjusted to ensure that patients had sufficient range to indicate the change in the attribute that would have the equivalent value as reducing invasiveness. Therefore, the exercises were not designed to directly elicit patients’ willingness to tolerate the actual change observed with TAVR.
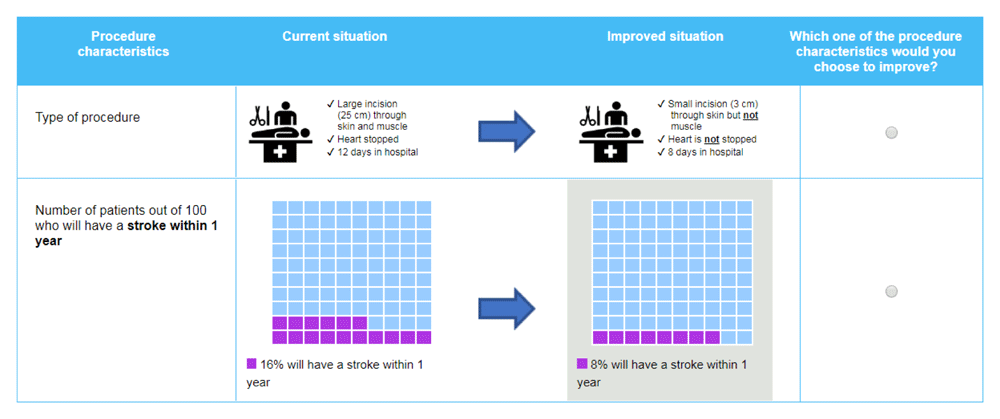
‘Type of Procedure’ was used in every pairwise comparison. Only the comparison attribute was varied across different attributes and performance levels.
Each set of pairwise comparisons included three iterations of the choice question. Depending on the answer to the choice question, the level of improvement offered on the non-procedure attribute was updated in the direction that made the value of improvements on the two attributes more similar than in the previous question. The algorithm used to identify participants’ indifference point included the levels used in each of the three iterations and how these changed dependent on the participants’ previous responses is shown in extended data, Appendix 124.
A pilot of the survey among five AS patients, carried out over a 4-week period in June 2018, ensured acceptable cognitive burden, clarity of the instructions, and ease of use of the elicitation software. Before completing the ASW exercises, participants were introduced to the attributes and their definitions. Participants also completed two ASW practice questions; interpretation of their response to these practice questions was tested to ensure participants understood how to complete each of the pairwise comparisons. Only eighteen participants (8.2%) incorrectly interpreted the meaning of their response to the first practice question, and no participants incorrectly interpreted their response to the second practice question. Each participant completed a proportion of the possible pairwise question—either 3 or 4 sets of pairwise comparison questions. Participants also completed clinical and demographic questions, and health literacy and numeracy questionnaires, available as extended data24.
Potentially eligible participants were recruited by M3 Global Research via email and from the membership of Heart Valve Voice and Mended Hearts patient organizations through e-mails and advertisements on social media platforms. Patients from the American Heart Association membership who had given prior permission to receive mailings were also invited to participate. Potential participants were directed to an online screening tool, where their eligibility for the survey was determined. Participants had to meet specific inclusion/exclusion criteria to participate (Box 1). There were no predefined enrollment targets or stratification by other demographic characteristics. Following completion of the online screening tool, eligible participants were directed to the web survey. They were also sent a link to the web survey, available as extended data24, via e-mail so that they were able to complete the study at a time that was convenient for them.
Inclusion criteria
Be at least 19 years of age
Have a self-reported diagnosis of aortic valve disease
Able to read and understand English
Willing and able to complete an online survey
Willing and able to provide (electronic) consent to participate in study
Be a resident of the United States
Have been treated procedurally (TAVR or SAVR) for AS in the past 10 years
or
Be willing and able to participate in a telephone interview, and to be audio-recorded (qualitative pilot only)
Exclusion criteria
Have a cognitive impairment, hearing difficulty, visual impairment, acute psychopathology
Have insufficient knowledge of English, which could interfere with the patient’s ability to provide written consent and complete the web survey
Are not experiencing at least one of the symptoms of AS, as described in the inclusion criteria
Participant responses to the ASW questions identified the level of change in an attribute that patients would be willing to accept in exchange for improving their procedure from ‘invasive’ to ‘minimally invasive.’ For instance, a participant might assign the same value to a 2% reduction in mortality risk as reducing the invasiveness of the procedure. Inverting this relationship, responses were used to estimate the maximum acceptable increase in risks (MIR) or the maximum acceptable reduction in benefits (MRB) that participants would be willing to tolerate in exchange for moving from an invasive procedure to a minimally invasive procedure. In the above example, the participant would be willing to tolerate a MIR of 2% in mortality risk to reduce the invasiveness of the procedure.
In cases of incomplete or missing data, no data imputation was performed, and unanswered questions were coded as missing. Data from participants who completed only a portion of the survey were included in the analyses if they had completed all the questions in at least one ASW exercise.
Participant preferences were incorporated into the following benefit-risk model constructed in Microsoft Excel.
Where
U(x) is the overall value generated by procedure x.
wi is the weight associated with attribute i,
vi is the partial value function for attribute i, which was assumed to be linear
xi is the performance of performance x on attribute i
Participants’ MIR/MRB was converted into an attribute weight (wi) by setting the weight for ‘type of procedure’ to 1, and then dividing the range in performance between TAVR and SAVR on that attribute (Table 2) by the MIR/MRB. For instance, if patients were willing to tolerate a 2% increase in mortality risk in exchange for a reduction in invasiveness, and reducing invasiveness is given a weight of 1, then the 4.8% change in mortality risk covered by the benefit-risk model (0.5%–5.3% range identified in Table 2) would be given a weight of 2.4 (4.8%/2%). Weights across all attributes in the model were then normalized to sum to 100.
Four outputs were generated from the benefit-risk model to evaluate patients’ preferences for TAVR and SAVR. First, the incremental overall value generated by TAVR:
Second, the incremental partial value on each attribute generated by TAVR:
Where
Third, a threshold analysis was undertaken, estimating the level of performance (minimum acceptable benefit or maximum acceptable risk) on each attribute that would leave patients indifferent between TAVR and SAVR. More specifically:
Where
MARa or MABa is the maximum acceptable risk or minimum acceptable benefit of attribute a
TAVRa is the performance of TAVR on attribute a (see Table 2).
x1 unit a is a single unit of performance on attribute a
Table 3 outlines the definitions of MIR, MRB, MAR, and MAB.
Fourth, a Monte Carlo Simulation (MCS) was run to explore uncertainty in model inputs. That is, the benefit-risk model was run 10,000 times for both TAVR and SAVR. In each instance, the model drew from the distribution around both performance and weight inputs. Specifically, performance inputs were drawn from the distribution around TAVR and SAVR performance data (Table 2). Weight inputs were drawn in a manner that reflected the probability that participants identified different MIR/MRBs in their responses to the survey. For each iteration of the MCS, TAVR and SAVR, we’ve ranked based on U (Equation 2) and the proportion of instances that TAVR ranked first was estimated.
In accordance with ethical practice, Institutional Review Board (IRB) approval was obtained through Advarra (approval MOD00300398 and MOD00354863) to comply with human participants research requirements prior to initiation of participant recruitment or administration of measures in the pilot or main studies. Informed consent was recorded electronically via the online survey platform. Any change to the protocol and/or informed consent form was resubmitted to the IRB for review and approval prior to implementation. The study was available for monitoring, auditing, IRB review, and regulatory inspection as applicable.
A total of 219 patients completed the survey over two rounds (Table 4). Raw data are available from Open Science Framework24. The majority of patients were less than 60 years old (n=132, 60.3%). More than half of the respondents were female (n=128, 58.4%) and a majority were white (n=173, 79.0%). Over half of the sample had completed a college degree or higher (n=157, 71.7%). Most of the participants lived with a partner/spouse, family, or friends (n=164, 74.9%). No participants reported experiencing severe limitations to their physical activity. Few patients demonstrated low health literacy (n=23, 10.5%) or numeracy (n=22, 10.0%), and only 7 patients (3.2%) were low on both scales.
| Characteristics | Patients, n (%) |
|---|---|
| Gender | |
| Male | 91 (41.6%) |
| Female | 128 (58.4%) |
| Ethnic background | |
| Not Hispanic or Latino | 209 (95.4%) |
| Age group, years | |
| 19–39 | 58 (26.5%) |
| 40–59 | 74 (33.8%) |
| 60–74 | 55 (25.1%) |
| 75–89 | 29 (13.2%) |
| 90+ | 3 (1.4%) |
| Racial background | |
| White | 173 (79.0%) |
| Black or African American | 27 (12.3%) |
| Asian | 13 (5.9%) |
| Native Hawaiian or other Pacific Islander | 3 (1.4%) |
| American Indian or Alaskan Native | 4 (1.8%) |
| Other | 7 (3.2%) |
| Living Situation | |
| Living alone | 54 (24.7%) |
| Living with a partner or spouse, family, or friends | 164 (74.9%) |
| Employment Status | |
| Employed, full-time | 93 (42.5%) |
| Employed, part-time | 28 (12.8%) |
| Homemaker | 8 (3.7%) |
| Student | 6 (2.7%) |
| Unemployed | 8 (3.7%) |
| Retired | 71 (32.4%) |
| Disabled | 19 (8.7%) |
| Highest level of education completed | |
| Secondary/high school | 16 (7.3%) |
| Some college | 46 (21.0%) |
| College degree | 93 (42.5%) |
| Postgraduate degree | 63 (28.8%) |
| Other: | 1 (0.5%) |
| General health within past week | |
| Very good | 55 (25.1%) |
| Good | 85 (38.8%) |
| Fair | 65 (29.7%) |
| Poor | 13 (5.9%) |
| Very poor | 1 (0.5%) |
| Previously treated for AS | |
| Yes | 176 (80.4%) |
| No | 43 (19.6%) |
| NYHA Classification | |
| No limitation of physical activity | 78 (35.6%) |
| Slight limitation of physical activity | 101 (46.1%) |
| Marked limitation in physical activity | 40 (18.3%) |
| Severe limitation in physical activity | 0 (0.0%) |
| Health literacy and numeracy | |
| Low literacy (1) | 23 (10.5%) |
| Low numeracy (2) | 22 (10.0%) |
| Overall low literacy / numeracy (3) | 7 (3.2%) |
1Responses were scored between 0 (always) and 4 (never). Each participants’ scored responses were averaged for a composite score ranging from 0–4. A low score if ≤2.
2Participants were given one point for each correctly answered question (maximum numeracy score = 5). A low score if given is ≤2 answered incorrectly. 3Overall low: individuals who scored low on both educational level and objective health literacy. NYHA, New York Heart Association.
When responding to the ASW questions, only a small proportion (8.68%) of participants ‘straight-lined’ on all questions—consistently choosing to improve either ‘procedure’ or the comparison attribute across all three iterations of the choice question. These responses may be a valid reflection of participants’ preference—suggesting a strong preference either for avoiding an invasive procedure, or a strong preference to prioritize improving other procedure attributes. Thus, these responses were included in the analysis. The impact of excluding these data were tested, and it was determined that results of the BRA were not sensitive to whether these data were included or excluded.
Table 5 shows the difference in performance of TAVR compared with SAVR on each attribute, and patients’ willingness to accept this difference in exchange for the lesser invasiveness of TAVR. The increase in risks or the reduction in benefits that patients are, on average, willing to accept in exchange for reducing procedure invasiveness is reported in the middle three columns. For instance, patients would be willing to tolerate a 6.69% increase in the probability of experiencing disabling, non-fatal stroke in exchange for the reduction in invasiveness associated with receiving TAVR instead of SAVR. In each case, patients were on average willing to accept TAVR’s performance on any attribute in exchange for its lower invasiveness. In the case of four attributes (mortality, disabling non-fatal stroke, independence, and dialysis), TAVR performs better than SAVR. Where SAVR performs better than TAVR (the need for new permanent pacemaker and time over which the procedure has been proven to work), patients would, on average, be willing to accept TAVR’s performance given its lower invasiveness. For example, participants are willing to tolerate a 6.98% increase in the risk of a new permanent pacemaker, while the probability of needing a new permanent pacemaker only increases by 3.3% with TAVR.
| Attribute | Impact of TAVR compared with SAVR† | Maximum Acceptable Increase in Risk/Maximum Acceptable Reduction in Benefit [Mean, (SD, n)] | Proportion of participants MIR/MRB > TAVR impact | |||
|---|---|---|---|---|---|---|
| Whole sample | <60yrs old | ≥60yrs old | p-value | |||
| Mortality (1 Month) | -2.9% | 3.86% (2.95, 109) | 3.4 (2.7, 65) | 4.5 (3.2, 44) | 0.0521* | 100% |
| Disabling Non-Fatal Stroke | -2.5% | 6.69% (5.73, 110) | 6.5 (5.6, 67) | 7.0 (6.0, 43) | 0.6524 | 100% |
| Independence | +23.0% | 13.94% (11.77, 131) | 13.5 (11.5, 80) | 14.6 (12.2, 51) | 0.5949 | 100% |
| New Permanent Pacemaker | +3.3% | 6.98% (5.71, 132) | 5.9 (5.1, 76) | 8.4 (6.2, 56) | 0.0121** | 69.8% |
| Requirement for Dialysis | -1.5% | 6.21% (5.62, 131) | 5.4 (5.1, 81) | 7.5 (6.2, 50) | 0.0351** | 100% |
| Proven to Work | -10yrs | 17.42 years (16.86, 131) | 17.9 (16.7, 80) | 16.6 (17.2, 51) | 0.6668 | 47.3% |
The standard errors in patients’ MIR/MRB suggests a substantial heterogeneity in patients’ responses to the ASW exercise (see extended data, Appendix S224 for more detail). Some of this heterogeneity was associated with participants’ age. MIR/MRB for three attributes—probability of having a new permanent pacemaker, probability of requiring dialysis, and one month mortality risk—were associated with whether patients are over or under 60 years old. Older patients were more willing to tolerate increases in risks/reductions in benefit to avoid having to undergo an invasive procedure.
No other correlation was found between participant characteristics and MIR/MRB. This includes whether a participant reported having previously undergone treatment for their AS. While this might be expected to influence preferences, the ability of the analysis to identify this influence is limited by the relatively small sample size and the small proportion of participants who reported not having previously received AS treatment (19.6%).
There were a large proportion of participants whose individual MIR/MRB was greater than the change in attribute performance achieved with TAVR (Table 5). For attributes where performance is better with TAVR compared to SAVR (mortality, disabling non-fatal stroke, independence, and dialysis), 100% of patients would prefer the improved performance and reduced invasiveness of TAVR. For the two attributes on which attribute performance is better with SAVR compared to TAVR, 70% of patients would be willing to accept the increased risk of needing a new permanent pacemaker, and 47% of participants would be willing to accept the shorter period for which TAVR had been proven to work in order to experience TAVR’s reduced invasiveness.
The above analysis compares the performance of TAVR on each attribute separately. A complete comparison of TAVR and SAVR should do so across all attributes simultaneously and take into account observed heterogeneity (in this case across age groups). This objective is accomplished by means of the benefit-risk model (see Equation 2 and Equation 3). Figure 2 and Figure 3 show the incremental value of TAVR (overall and by attribute) observed among patients 60 years old or older (Figure 2) and among patients less than 60 years old (Figure 3). These figures reveal that, overall, TAVR is of greater value to patients than SAVR. Specifically, patients placed greater value on TAVR based on a lower short-term mortality rate, reduced procedural invasiveness, and a quicker time to return to normal quality of life (independence) offsetting the value patients placed on longer period over which the procedure has been proven to work and reduced risk of needing a pacemaker generated by SAVR. Similar patterns were observed among younger and older patients.
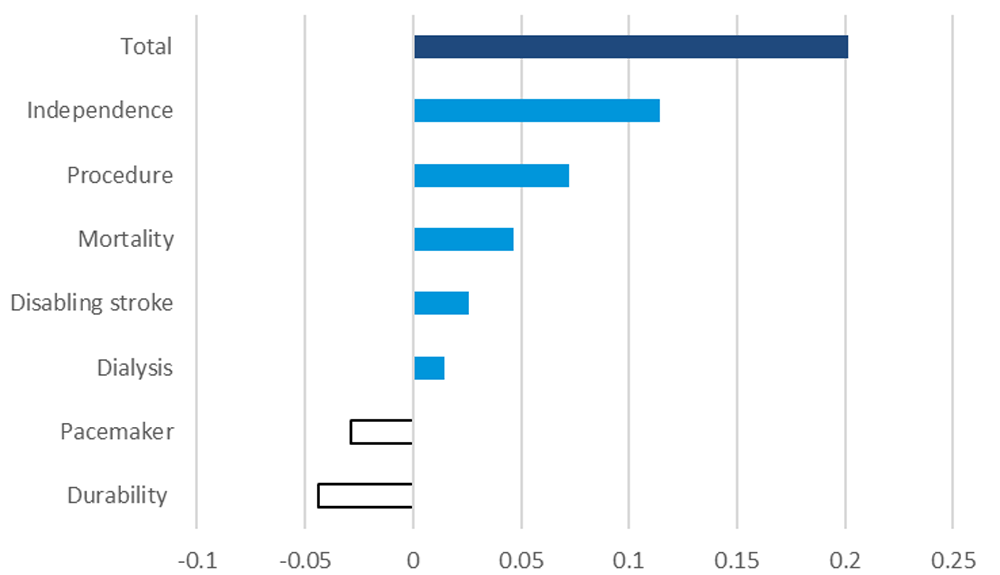
Table 6 reports a threshold analysis, which shows the minimum amount of benefit that patients would accept before preferring TAVR, or the maximum amount of risk that patients would tolerate before preferring TAVR. For example, given the incremental value that patients attach to TAVR (as reflected in Figure 2 and Figure 3) they would be willing to tolerate a mortality risk of 12.6% following TAVR before they would be indifferent between TAVR and SAVR.
The MCS shows that 79.5% of patients would prefer TAVR over SAVR. When the analysis is run separately for patients less than and greater than 60 years old, the proportions of patients preferring TAVR are 80.8% and 78.2% respectively. Removing patients who ‘one-lined’ in response to ASW exercises does not impact the results of the MCS, with 80.7% of patients still preferring TAVR.
The choice between TAVR and SAVR for the treatment of AS involves making trade-offs between: procedure invasiveness; the period over which the procedure has been proven to be effective; mortality, stroke and independence outcomes; and risks such as the need for a new pacemaker or dialysis. This study elicited patients’ preferences for AS procedure attributes to determine how they make these trade-offs, and thus whether they prefer TAVR or SAVR. Results suggest that, given the potential benefits and risks of TAVR and SAVR, on average, patients attach more value to TAVR, and the majority of patients would prefer TAVR. Patients placed a greater value on the lower invasiveness, quicker speed of recovery, and reduced risk of mortality, stroke and need for dialysis associated with TAVR than they did on longer period over which the procedure has been proven to work and reduced risk of needing a pace maker associated with SAVR.
Current guidelines from the American Heart Association for the procedural treatment of AS do not take into account recent clinical data supporting the use of TAVR25. Based on the recent clinical results of TAVR and the findings of this study, regulators may reach different conclusions about the need to protect patients from risks historically associated with TAVR. For instance, TAVR may not result in the increased risk of stroke that regulators might expect it to, and patients may be willing to tolerate the greater need for a permanent pacemaker in order to experience the benefits of TAVR.
The BRA revealed substantial heterogeneity in patient preferences for AS treatment. Some preference heterogeneity is explained by patient age, with older patients being less willing to tolerate the invasiveness of SAVR, instead preferring to accept greater potential risks associated with other procedure attributes in order to reduce the invasiveness. However, preference heterogeneity raises concerns about the attendance of participants to the preference elicitation tasks. A small proportion (8.68%) of participants straight-lined on all questions. While this might indicate a lack of attendance, it may also capture strong preferences for/against the invasiveness of SAVR. Furthermore, all participants interpreted their response to the practice questions correctly, and only a small proportion of respondents demonstrated low health literacy or numeracy. This provides some reassurance that the preference heterogeneity observed in this study reflects a genuine difference in preference, rather than being the result of patients failing to complete the survey in a meaningful way.
Two other studies that used ASW to elicit patient preferences have been published26,27. Both studies also observed substantial preference heterogeneity. One of these studies27 provided evidence supporting the validity and reliability of the preference outputs, both by replicating the results of the ASW with a thresholding exercise, and by comparing participants’ responses with their qualitative statements on the basis for their answers. This provides some reassurance about the validity of responses to the ASW exercise used for the current study. This may suggest that methods such as ASW, which elicit individual-level patient preferences, capture more preference heterogeneity than population-level methods, such as discrete choice experiments. Further work could usefully continue to validate the results of ASW exercises and test the hypothesis that individual-level preferences method captures greater heterogeneity in patient preferences.
Only one other study of AS patients’ treatment preferences has been published to date28. The study design was sufficiently different to the current study—focusing on patients’ willingness to accept the mortality risk associated with interventions—that it is not possible to directly compare the results. However, the study by Hussain et al.28 did reveal a higher risk tolerance among patients with greater disease burden (defined as weekly incidence of restricting symptoms, perceived change in health compared with 1 year earlier, EQ-VAS scores, and the New York Heart Association (NYHA) classification). Our study failed to identify an association between patient preferences and NYHA classification, though this might be due to the limited sample size and the small proportion of the sample in the more severe stages of the NYHA classification. Further research could usefully gather data from a larger sample of AS patients to determine the association of preferences and patient characteristics, such as NYHA classification or whether patients have previously undergone treatment for AS.
While a majority of patients in the current study preferred TAVR, a number of patients (around 20%) preferred SAVR. This, and the underlying heterogeneity in patient preferences, support the need for a shared decision-making (SDM) tool that will help patients and surgeons choose procedures based on both clinical indications and patient risk tolerance. The Patient-Centered Outcomes Research Institute (PCORI) has developed a SDM tool to support patients choose between SAVR and TAVR15. However, this tool includes a narrower range of treatment attributes—stroke risk, mortality risk and discharge home—than those included in the analysis reported in this study. Furthermore, the tool does not include a component to elicit a patient’s preferences.
As always, the conclusions of the study should be drawn in light of its limitations. First, it was not possible to engage patients in the design of the elicitation exercise. Instead, the patient voice was reflected in the design through a review of the limited preference research undertaken with AS patients to date and through the engagement that the experts who were consulted had themselves had with patients. Further work would usefully confirm with patients that no attributes were excluded from the study. Second, in the first survey round we relied on patient self-report of their AS diagnoses and severity, and in the second wave providing confirmation of AS diagnosis was voluntary. Third, the sample is healthier and younger than the population currently eligible for TAVR and SAVR29. AS patients were recruited from the membership of Heart Valve Voice and Mended Hearts as well as through M3 Global Research, and it is possible that patients who are motivated to join these organizations may have different preferences than the broader population. Further AS patient preference research should replicate this study, including older patients with more severe disease burden. Fourth, it was necessary to assume that partial value functions were linear. The sample size and the number of attributes for which preferences were being collected meant collecting data on the shape of the partial value functions would have overburdened respondents.
Finally, the application of ASW in BRA is relatively novel and raises a number of questions. First, it relies on the idea that patients who are indifferent between adding one or another feature to a good or service would be willing to accept either improvement. The concept of indifference is commonly evoked in methods to assess the value of changes in health outcome. Further, the baseline against which improvements are assessed is the worse level of performance of the treatments being evaluated and over 80% of patients had previously been treated for AS. Thus, in most cases it might be reasonable to assume that patients would accept the changes, as they represent outcomes better than those they’ve already accepted. However, to infer that they would be willing to accept improvements in outcomes assumes that they were well informed when they made these treatment choices. Further research should test participants willingness to accept changes in attributes.
Second, unlike some other elicitation methods, such as discrete choice experiments, the study assumes that respondents don’t make mistakes when they are giving their answers, which is unlikely if respondents are near to their indifference points. It is thus necessary to assume that any such mistakes average out when assessing preferences for the study sample.
Third, as all respondents start their evaluation of attributes at the same point, ASW may be subject to anchoring. To mitigate this risk, ‘worst’ levels in the choice tasks were defined as the ‘worst’ clinically relevant level. However, in some instances this may have introduced a ceiling effect, with insufficient improvement in the attribute available to identify the indifference point. In these instances, it was necessary to increase the ‘worst’ level beyond that experienced by patients. Further work could usefully explore this apparent tension between anchoring and ceiling effects inherent within ASW.
Most AS patients are willing to tolerate sizable increases in clinical risk in exchange for the benefits associated with TAVR. A BRA incorporating data from patients’ preferences for the attributes of AS treatments revealed a strong preference for TAVR compared to SAVR. The analysis also revealed substantial heterogeneity in individual patient preferences, partly associated with patient age. Further work is required to understand this heterogeneity, and whether additional patient characteristics such as NYHA class are associated with different preferences. In the meantime, SDM tools should incorporate the factors identified in this model to assist patients and clinicians in achieving a more patient-centered treatment decision.
Open Science Framework: AS patient preference data. DOI 10.17605/OSF.IO/UGD8X24.
This project contains the following underlying data:
Open Science Framework: AS patient preference data. DOI 10.17605/OSF.IO/UGD8X24.
This project contains the following extended data:
TAVR Manuscript Appendices S1_S2_Updated.docx (contains S1 Appendix, including S1 Table 1 and legends for S1 Figures 1-6; and S2 Appendix, including legends for S2 Figures 1-6).
TAVR Manuscript Figures_Updated.docx (S1 Figures 1-6 and S2 Figures 1-6).
TAVR Survey Contents_Updated.docx (a copy of the questionnaire given to each participant).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors would like to thank Heart Valve Voice and Mended Hearts for their support with the design of the survey, and their support recruiting patients.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
References
1. Determann D, Gyrd-Hansen D, de Wit GA, de Bekker-Grob EW, et al.: Designing Unforced Choice Experiments to Inform Health Care Decision Making: Implications of Using Opt-Out, Neither, or Status Quo Alternatives in Discrete Choice Experiments.Med Decis Making. 39 (6): 681-692 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Health preference research and the use of these preference measures to infer risk tolerance.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Internal medicine physician with prior experience in outcomes research and shared decision making. Not actively involved in research in my present role as a venture capitalist, but I led the MDIC Patient Centered Benefit-Risk Project that developed a framework for incorporating patient preferences into FDA medical device regulation and developed the first catalog of patient preference methodologies included as an appendix to that report.
Competing Interests: I have received honoraria from Edwards LifeSciences.
Reviewer Expertise: shared decision making
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: I have received honoraria from Edwards LifeSciences for speaking on shared decision making.
Reviewer Expertise: shared decision making
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: I was asked to speak to the senior management of Edwards Lifesciences, which provided the grant for this study, in August 2017 about the use of patient preference information in FDA regulatory approval of medical devices. I was not paid for the talk, but was reimbursed travel expenses. Additionally, one of the authors, Barry Liden, has served with me on the Steering Committee for the MDIC Patient Centered Benefit Risk (PCBR) Project, where I was the MDIC Board member leading that project. Barry now chairs the MDIC Science of Patient Input (SPI) Steering Committee, where I am also a member.
Reviewer Expertise: Internal medicine physician with prior experience in outcomes research and shared decision making. Not actively involved in research in my present role as a venture capitalist, but I led the MDIC Patient Centered Benefit-Risk Project that developed a framework for incorporating patient preferences into FDA medical device regulation and developed the first catalog of patient preference methodologies included as an appendix to that report.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 5 (revision) 08 Feb 21 |
read | ||
|
Version 4 (update) 05 May 20 |
read | ||
|
Version 3 (revision) 21 Jun 19 |
|||
|
Version 2 (revision) 14 May 19 |
read | read | |
|
Version 1 08 Apr 19 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)