Keywords
Line transect, fish visual census, unit of artificial reef, fish species, Chaetodontidae, Gusung Batu Lampe, Mahakam Delta, Kutai Kartanegara
This article is included in the ICTROPS 2018 collection.
Line transect, fish visual census, unit of artificial reef, fish species, Chaetodontidae, Gusung Batu Lampe, Mahakam Delta, Kutai Kartanegara
Artificial reefs, especially in East Kalimantan, are rarely utilized despite their ability to create new habitats. Nowadays, artificial reefs had been tried by the province of East Kalimantan at a particular area to rehabilitate fish habitat degradation, such as at Gusung Batu Lampe in Pangempang water, Kutai Kartanegara district. The degradation of coral reefs in the area was primarily caused by the practices of bottom trawling and blast fishing. In 2011 and 2012, the condition of coral reefs in Pangempang water was reported for the first time by Suyatna et al. (2017a), and convinced that the live coral (LC) form was recorded in only 21.0% of the area of 41.84 ha. Pangempang water belongs to the Mahakam delta which homes many of fish species. Suyatna et al. (2010) identified 43,340 fish caught by minitrawl and noticed 131 species from 87 genera, 61 families and 10 orders. A total of 13 species were observed in Mahakam river 40 km behind the river mouth (Suyatna et al., 2017b), and among of them, the longfin anchovy Setipinna sp., was found 230 km from the coastline (Suyatna et al., 2017c). Fish found in the Mahakam delta were also recognized along the coastal waters of East Kalimantan. At least 22 families from 8,291 fish were also observed at the industrial estate in Bontang, 75 km north of the delta (Suyatna et al., 2016) and 29 species in the coastal water of East Kutai district (Juliani & Suyatna, 2014).
However, after artificial reef construction, scientific information describing fish colonization factors associated with the reef, such as fish species and fish richness remains unstudied. From the beginning, the reef is expected to restore fish populations in the area, and therefore a preliminary study is urged to be carried out for a certain duration. For this reason, this study was performed to emphasize the importance and benefits of constructing artificial reef and to identify and analyze the fish community structure associated with the reef.
An underwater line transect and fish visual census (FVC) were applied in this survey. FVC method is commonly used at site (Lim, 2017) and line transect limits the observation area to only 200 m2 (20 m long and 10 m wide, 5 m to the right and 5 m to the left), measured using a 50 m roll meter. The distance of the observation area was around 5 km from the coast at the coordinate of 0° 13’02.1” S 117° 29’35.1” E and a 40 HP speed boat was used to transport divers to the site. The artificial reef structure comprised 34 units of tetrahedral concrete block, sizing 60 cm x 60 cm at base, 60 cm high and 35 cm x 35 cm wide on top, with four holes in each block, were placed in a row. Two SCUBA divers descended to the bottom; one ensured the safety of the divers and the other one took photographs while counting the number of fish. Fish photographs were realized with Action Kogan 4K and Nikon AW 130 underwater cameras. These cameras were also used to record the vicinity condition such as a unit of reef, and a GPS device (Garmin) was used to determine the observation site. All observational data were recorded using underwater diving slates. Observation took place for only 30 to 45 minutes in the morning at 09:00, in the early afternoon at 13:00 and at the late afternoon at 17:00, with the total duration of 90 to 135 minutes for one survey. Surveys were performed four times: in November and December 2016, and in January and July 2017.
Recording and taking photographs of fish started about 20 minutes after transect was laid to provide time for fish to react to the natural habitat. Fish species identification was conducted with reference to Allen (2000); Anam & Mostarda (2012); Masuda et al. (1975); Peristiwadi (2006).
Water physical parameters such as transparency, salinity and temperature were measured with water quality meter AZ 8603 (Shenzhen Hengkaituo Sci-Tech Co., Ltd, Guandong, China). Water velocity was assessed by drifting on the surface a small buoy for 10 m distance and noting the time to pass was using a stopwatch. Water depth was measured with a measuring rope.
Palaeontological Statistics version 3.20 (Hammer et al., 2001) was used to analyze the diversity indices, namely dominance, Shannon’s H, evenness, and Margalef species richness index. Bray–Curtis multivariate statistics, which is known to be more effective than other analytical tools (Kurt & Merlyn, 1998) such as Euclidean, Morisita, Mahalanobis, and Jaccard statistics, was applied to analyze the similarity of fish size colonization among observation times. Fish density (individual/m2) was calculated manually.
Taxonomically, 38 species belonging to 26 genera and 16 families were observed during surveys. A total of 25 species and 12 families were observed in the first survey; 22 species and 9 families in the second survey; 26 species and 11 families in the third survey; and 23 species and 13 families in the last survey (Table 1 and Table 2). All of the fish were small-bodied. In each survey, the two families Pomacentridae and Labridae were families with the most common and adaptable species as well as the most abundant in individual number, followed by the Chaetodontidae and Acanthuridae families. The remaining 12 families were only represented by one or two species. However, the four families Chaetodontidae, Siganidae, Caesionidae and Nemipteridae were present in every survey. Some families were only present in single surveys. The fish species observed at the fourth survey to represent the fish colonization of the artificial reef at Gosong Batu Lampe are shown in Figure 2.
| Family and species* | Survey 1 (Nov 2016) | Survey 2 (Dec 2016) | ||||
|---|---|---|---|---|---|---|
| 09:00 | 13:00 | 17:00 | 09:00 | 13:00 | 17:00 | |
| Labridae (1) | ||||||
| Thalassoma lunare (1) | 7 | 14 | 3 | 20 | 7 | 11 |
| Halichoeres hortulanus (2) | 2 | 0 | 0 | 3 | 0 | 0 |
| H. timorensis (3) | 2 | 1 | 0 | 1 | 0 | 1 |
| Cheilinus chlorourus (4) | 0 | 1 | 0 | 1 | 0 | 0 |
| Labroides dimidiatus (5) | 0 | 3 | 1 | 3 | 1 | 0 |
| Scarus rubroviolaceus (6) | 1 | 0 | 0 | 3 | 2 | 0 |
| Acanthuridae (2) | ||||||
| Acanthurus nigrofuscus (1) | 0 | 1 | 0 | 4 | 5 | 3 |
| A. nigricanda (2) | 0 | 0 | 0 | 0 | 0 | 0 |
| A. bariene (3) | 0 | 0 | 0 | 0 | 0 | 0 |
| A. grammoptilus (4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Pomacanthidae (3) | ||||||
| Centropyge tibicen (1) | 1 | 0 | 1 | 0 | 0 | 0 |
| Pomacentridae (4) | ||||||
| Amphiprion ocellaris (1) | 3 | 3 | 3 | 3 | 3 | 3 |
| Cromis weberi (2) | 1 | 1 | 3 | 1 | 1 | 1 |
| C. xanthochira (3) | 0 | 0 | 3 | 4 | 3 | 0 |
| Dascyllus trimaculatus (4) | 2 | 2 | 17 | 10 | 19 | 2 |
| D. reticulates (5) | 22 | 19 | 14 | 30 | 3 | 12 |
| Pomacentrus amboinesis (6) | 1 | 1 | 0 | 2 | 1 | 2 |
| P. mollucensis (7) | 1 | 4 | 4 | 2 | 0 | 2 |
| P. chrysurrus (8) | 0 | 0 | 0 | 0 | 0 | 0 |
| Plectrogliphidodon lacrymatus (9) | 1 | 0 | 1 | 4 | 1 | 0 |
| Chaetodontidae (5) | ||||||
| Chaetodon vegabundus (1) | 0 | 0 | 4 | 1 | 0 | 0 |
| Chaetodon kleinii (2) | 1 | 1 | 1 | 4 | 1 | 2 |
| Chaetodon lunulatus (3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Tetraodontidae (6) | ||||||
| Canthigaster papua (2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Gobiidae (7) | ||||||
| Amblyeleotris periophthalma (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| A. guttata (2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Rigilius goby (3) | 1 | 1 | 0 | 0 | 0 | 0 |
| Mullidae (8) | ||||||
| Upeneus tragula (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Siganidae (9) | ||||||
| Siganus virgatus (1) | 2 | 2 | 2 | 2 | 2 | 2 |
| S. punctatus (2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Caesionidae (10) | ||||||
| Caesio teres (1) | 0 | 0 | 5 | 0 | 18 | 0 |
| Nemipteridae (11) | ||||||
| Scolopsis ciliata | 1 | 3 | 5 | 5 | 0 | 1 |
| Scorpaenidae (12) | ||||||
| Pterois mombasae (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Serranidae (13) | ||||||
| Cephalophosis boenak (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Lutjanidae (14) | ||||||
| Lutjanus decussates (1) | 0 | 0 | 1 | 3 | 0 | 0 |
| Zanclidae (15) | ||||||
| Zanclus cornutus (1) | 2 | 1 | 2 | 1 | 0 | 3 |
| Lethrinidae (16) | ||||||
| Lethrinus harak (1) | 0 | 0 | 1 | 0 | 0 | 0 |
| Number of fish | 51 | 58 | 71 | 107 | 67 | 45 |
| Number of taxa | 17 | 16 | 18 | 21 | 14 | 13 |
| Family and species* | Survey 3 (Jan 2017) | Survey 4 (July 2017) | ||||
|---|---|---|---|---|---|---|
| 09:00 | 13:00 | 17:00 | 09:00 | 13:00 | 17:00 | |
| Labridae (1) | ||||||
| Thalassoma lunare (1) | 3 | 6 | 2 | 6 | 7 | 4 |
| Halichoeres hortulanus (2) | 3 | 2 | 0 | 2 | 2 | 2 |
| H. timorensis (3) | 0 | 2 | 2 | 2 | 3 | 0 |
| Cheilinus chlorourus (4) | 2 | 2 | 2 | 2 | 0 | 0 |
| Labroides dimidiatus (5) | 1 | 3 | 2 | 0 | 0 | 0 |
| Scarus rubroviolaceus (6) | 0 | 1 | 0 | 0 | 0 | 0 |
| Acanthuridae (2) | ||||||
| Acanthurus nigrofuscus (1) | 6 | 3 | 2 | 3 | 4 | 2 |
| A. nigricanda (1) | 0 | 0 | 0 | 3 | 2 | 3 |
| A. bariene (2) | 0 | 0 | 0 | 0 | 2 | 0 |
| A. grammoptilus (3) | 3 | 1 | 0 | 0 | 0 | 0 |
| Pomacanthidae (3) | ||||||
| Centropyge tibicen (1) | 0 | 3 | 0 | 2 | 2 | 2 |
| Pomacentridae (4) | ||||||
| Amphiprion ocellaris (1) | 3 | 3 | 3 | 0 | 0 | 0 |
| Cromis weberi (2) | 3 | 2 | 0 | 6 | 6 | 3 |
| C. xanthochira (3) | 2 | 0 | 0 | 0 | 0 | 0 |
| Dascyllus trimaculatus (4) | 11 | 11 | 14 | 9 | 6 | 9 |
| D. reticulates (5) | 5 | 27 | 24 | 11 | 7 | 8 |
| Pomacentrus amboinesis (6) | 4 | 2 | 1 | 7 | 8 | 5 |
| P. mollucensis (7) | 2 | 3 | 4 | 0 | 0 | 0 |
| P. chrysurrus (8) | 0 | 0 | 0 | 4 | 4 | 2 |
| Plectrogliphidodon lacrymatus (9) | 2 | 2 | 1 | 0 | 0 | 0 |
| Chaetodontidae (5) | ||||||
| Chaetodon vegabundus (1) | 1 | 2 | 0 | 2 | 0 | 2 |
| Chaetodon kleinii (2) | 2 | 3 | 1 | 1 | 1 | 1 |
| Chaetodon lunulatus (3) | 0 | 4 | 0 | 0 | 0 | 0 |
| Tetraodontidae (6) | ||||||
| Canthigaster papua (2) | 0 | 0 | 0 | 2 | 2 | 0 |
| Gobiidae (7) | ||||||
| Amblyeleotris periophthalma (1) | 0 | 0 | 0 | 0 | 3 | 2 |
| A. guttata (2) | 0 | 1 | 0 | 0 | 2 | 0 |
| Rigilius goby (3) | 0 | 3 | 2 | 0 | 0 | 0 |
| Mullidae (8) | ||||||
| Upeneus tragul (1) | 0 | 0 | 0 | 0 | 2 | 0 |
| Siganidae (9) | ||||||
| Siganus virgatus (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| S. punctatus (1) | 0 | 2 | 2 | 2 | 0 | 2 |
| Caesionidae (10) | ||||||
| Caesio teres (1) | 4 | 0 | 0 | 27 | 64 | 4 |
| Nemipteridae (11) | ||||||
| Scolopsis ciliate (1) | 5 | 2 | 2 | 4 | 4 | 3 |
| Scorpaenidae (12) | ||||||
| Pterois mombasae (1) | 0 | 1 | 0 | 0 | 0 | 0 |
| Serranidae (13) | ||||||
| Cephalophosis boenak (1) | 0 | 0 | 0 | 3 | 2 | 0 |
| Lutjanidae (14) | ||||||
| Lutjanus decussates (1) | 0 | 0 | 0 | 0 | 2 | 1 |
| Zanclidae (15) | ||||||
| Zanclus cornutus (1) | 1 | 2 | 0 | 0 | 0 | 0 |
| Lethrinidae (16) | ||||||
| Lethrinus harak (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of fish | 63 | 93 | 64 | 98 | 135 | 55 |
| Number of taxa | 18 | 25 | 15 | 19 | 21 | 17 |
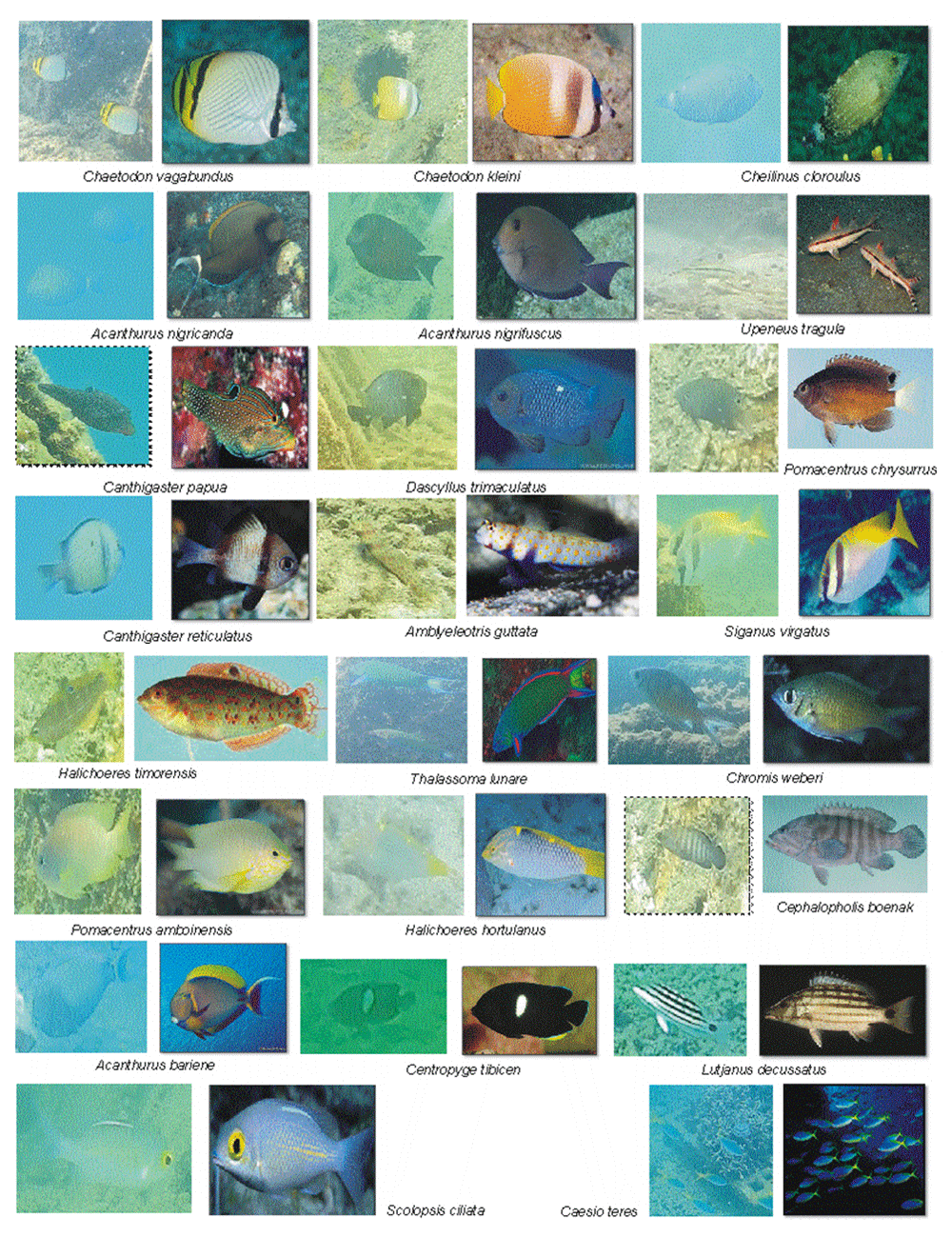
Mallet et al. (2016) examined reef fish assemblages during daylight hours, 10 times a day for 34 consecutive days in a branching coral and they discovered fish abundance and taxa richness were greater in the early morning, in which the most frequent observed families was Pomacentridae and Labridae (in 100% and 99.6% of cases, respectively). In our study these two families were also encountered in every survey, and were the most specious and populous families, followed by Acanthuridae and Chaetodontidae families. Honda et al. (2013) reported that the most dominant families on the basis of species number in coral reefs were Labridae, Pomacentridae and Chaetodontidae. There were nine families identified in all surveys, but with only one species in each family, and according to Hernández-Velasco et al. (2016) the families distributed in tropical regions inhabit shallow coral reefs. Chaetodon kleinii and two other species of Chaetodontidae identified in our study indicated that Gusung Batu Lampe has potentiality to attract more coral reef fish. Chaetodon sp. are known to be obligate corallivores, which feed entirely on coral polyps (Kulbicki et al., 2011; Yusuf & Ali, 2004), and rely on reefs for breeding, nursing and shelter (Muhammad et al., 2017). In term of single species, Caesio teres, of the family Caesionidae (Fusilier), was the most abundant species and fed primarily on plankton; this species is included commercially as an important food fish. Some species of this family are distributed widely in mid-water over reefs (Bawole et al., 2014) and are utilized as bait fish to catch Tuna (Rajasuriya, 2014). Acanthurus, of the family Acanthuridae, commonly known as surgeonfish, was encountered in this study as the third major group. This group feeds upon algae.
Based on the Bray–Curtis index, the number of fish at 09:00 in the morning and at 13:00 in the early afternoon was 95.0% similar, but declining less to 80.0% at 17:00 in the late afternoon (Table 3). This phenomenon of retreat of coral fish in the late afternoon could be an effect of light intensity (Rickel & Genin, 2005). Light may influence and increase water temperature (Mcleod et al., 2013), and such condition could affect the performance of coral reef fish, said otherwise they which tend to harbor or hide in reef holes and unseen (Suyatna et al., 2016). Likewise, the number of taxa between two observation times was 99.0% similar, but the similarity in the late afternoon was only 91.0%.
| Time | Mean density | Mean taxa | ||||
|---|---|---|---|---|---|---|
| 09:00 | 13:00 | 17:00 | 09:00 | 13:00 | 17:00 | |
| 09:00 | 1 | 0.949405 | 0.848375 | 1 | 0.993377 | 0.913043 |
| 13:00 | 0.949405 | 1 | 0.799320 | 0.993377 | 1 | 0.906475 |
| 17:00 | 0.848375 | 0.799320 | 1 | 0.913043 | 0.906475 | 1 |
Fish density in a unit area (individual/m2) increased from the first to the last survey, from 1/4.444 m2 to 1/1.481 m2 (Table 4). This indicates that the reef at Gusung Batu Lampe potentially provide a valuable area forcoral reef fish population since the availability of refuge sites of reefs may increase fish number (Grossman et al., 1997), and reef size did not significantly affect the density of colonists (Borntrager & Farrell, 1992).
The diversity index was applied to describe regarding of how many different species are present over the surveys among the observation times. This study revealed that the number of colonists, species richness and species diversity was highest in at 09:00 in the morning and at 13:00 in the afternoon, and the highest species richness arose in the 3rd survey, particularly at 13:00 (Table 5). The other diversity indices showed a different population sizes of the colonists during this study.
Some relevant water quality parameters measured at the location showed favorable water conditions for fish to live (Table 6). This is notable, since water quality factors such as current velocity may affect food intake (Belal, 2015), water transparency determines underwater visibility and fish assemblage (Enrique de Melo et al., 2009), and temperature and salinity may decrease species richness and fish abundance (Brucet et al., 2012). Fish can live in various places, but they often occupy a particular place and certain depth (Costa et al., 2013).
Overall, 38 fish species from 16 families were successfully identified during surveys. Distribution of taxa and colonists were more frequently occurred at 09:00 and 13:00 than at 17:00. In 4 months of observation, fish density increased from 1/4.444 m2 to 1/1.481 m2 indicating the increase of fish colonization size. The most populous families were Pomascentridae and Labridae, and the obligate corallivore discovered was Chaotodon kleinii. The artificial reef should be elevated or extended to provide a more available water column for fish to be more colonized.
Dataset 1. Raw water quality data for each survey. DOI: https://doi.org/10.5256/f1000research.16736.d226152 (Suyatna et al., 2018).
Authors acknowledge the Islamic Development Bank (IDB) for part financial support (the last survey; IDR. 53,000,000) as stated in the Decree of Mulawarman University Rector No 448/SK/2017, dated 16 March 2017.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Faculty of Fisheries and Marine Science Mulawarman University for allowing the use of laboratory facilities, Head of the Surveillance Group of Local Community in Muara Badak: Muhammad Mansyur, Assistance of the Hydro-oceanography Laboratory Muhammad Raafi and all students involved in the field and laboratory work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Bohnsack JA, Sutherland DL: Artificial Reef Research: A Review with Recommendations for Future Priorities. Bulletin of Marine Science. 1985; 37 (1). Reference SourceCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: artficial reef materials and ecology, seagrass, invasive species
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 09 Jan 19 |
read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)