Keywords
Mitogenome, mtDNA , adaptive evolution, positive selection, Etheostoma, Percina
Mitogenome, mtDNA , adaptive evolution, positive selection, Etheostoma, Percina
Introduction was expanded to include information about darters and their adaptation to different niches that may select for OXPHOS variants. Also, table 3 was added to specify types of substitutions identified as being under positive selection and the results and discussion section was expanded to incorporate this addition. Table 3 was added to address the suggestion regarding the identity of amino acid substitutions
See the authors' detailed response to the review by Dusan Kordis
Mitochondria provide cells with nearly all energy as a result of oxidative phosphorylation (OXPHOS). Vertebrate mitochondria are primarily maternally inherited and contain mitochondrial DNA (mtDNA), which contains 13 protein coding genes, the products of which contribute, along with nuclear-encoded proteins (Sunnucks et al., 2017), to the formation of the mitochondrial OXPHOS machinery (Ingman & Gyllensten, 2006; Ladoukakis & Zouros, 2017). While purifying selection acts on deleterious mtDNA mutations (Burr et al., 2018), James et al. (2016) utilized a variety of McDonald-Kreitman type analyses of a large number of animal species to show that mtDNA evolution is dominated by slightly deleterious mutations but also undergoes a significant amount of adaptive evolution. Several studies of the evolution of mtDNA-encoded mitochondrial protein coding genes both within fish species (Consuegra et al., 2015; Teacher et al., 2012) and between fish species (D’Anatro et al., 2017; Garvin et al., 2011; Zhang & Broughton, 2015) identified positive selection acting on some of these genes.
Darters are a group of approximately 200 small, benthic perch-like fish species that are found in streams and rivers in eastern North America (Near et al., 2011). Various species are adapted to fast water or slow water habitats that can differ in oxygen content, especially in the summer, and these species differ in their metabolic rates (Kist, 2016; Ultsch et al., 1978). Adaptation to such environmental niches may include adaptive changes to the OXPHOS machinery and thus this study utilizing all currently available mitochondrial genome sequences of darter species was carried out to determine if positive selection can be detected in any of the mitochondrial protein coding genes.
All mitochondrial genome sequences utilized in this study were obtained from GenBank except those from Etheostoma chuckwachatte, Etheostoma jessiae, Etheostoma spectabile, Etheostoma tallapoosae and Percina crypta. The E. spectabile sequence was obtained from Rachel Moran, University of Illinois. Whole genome sequencing was performed on E. chuckwachatte, E. jessiae, E. tallapoosae and P. crypta genomic DNA (purified from fin/muscle tissue with the Qiagen DNeasy Blood and Tissue Kit) by the Georgia Genomic Facility at the University of Georgia utilizing the Illumina NextSeq (PE 150) or MiSeq (PE 250) sequencer. A subset of sequence reads from each species, sufficient for at least 30-fold coverage, was aligned to one of the GenBank obtained darter mitochondrial genomes utilizing the Map to Reference function in Geneious 9.1.8 software (Biomatters Ltd., Auckland, New Zealand). Annotated darter mitochondrial genome sequences from GenBank were aligned with the newly assembled darter mitochondrial genome sequences and annotations were transferred and manually adjusted where necessary with Geneious software.
Phylogenetic analysis was carried out on all 13 concatenated and aligned mitochondrial protein coding sequences of the 11 darter species as well as of Perca flavescens, Sander vitreus and Anarhichas minor (outgroup). Bayesian inference (BI) analysis was performed with MrBayes (Ronquist & Huelsenbeck, 2003), as implemented in Geneious software but utilizing optimal partitioning and optimal substitution models determined by PartitionFinder v2.1.1 (AICc, greedy algorithm, MrBayes specific models) (Guindon et al., 2010; Lanfear et al., 2012; Lanfear et al., 2017). The rooted darter sub-tree (Figure 1) was extracted with MEGA 7.0.26 software (Kumar et al., 2016). Aligned darter mitochondrial protein coding genes were subject to site-specific tests for positive selection utilizing the Selecton implementation of an empirical Bayes approach (Doron-Faigenboim et al., 2005; Stern et al., 2007) and also the MEME and FEL methods implemented on the Datamonkey webserver (Delport et al., 2010). The darter tree was used in all three of these analyses.
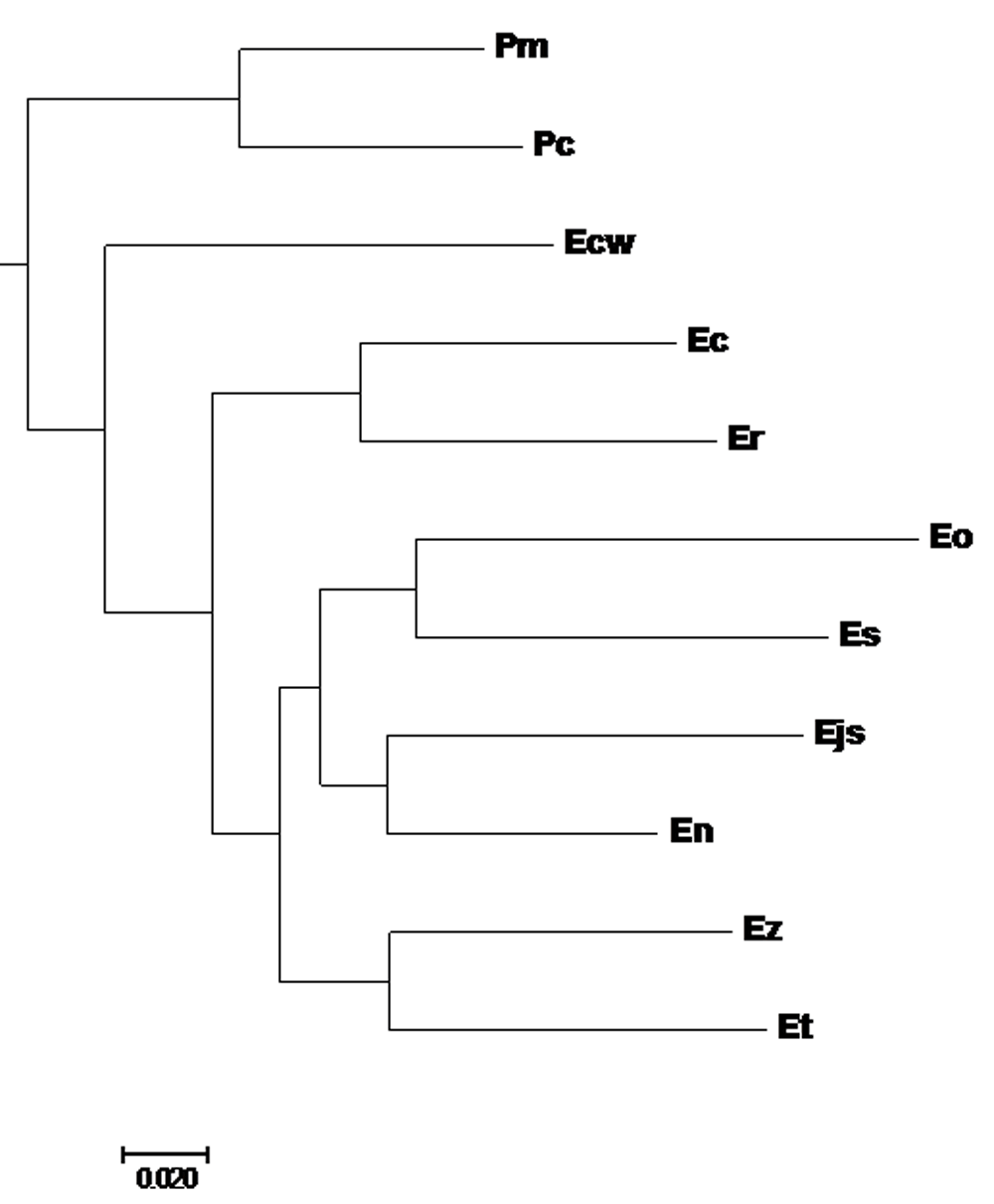
Branch lengths measured in number of substitutions per site. Posterior probabilities of all branches are greater than 0.91. Pm, Percina macrolepida; Pc, Percina crypta; Ecw, Etheostoma chuckwachatte; Ec, Etheostoma caeruleum; Er, Etheostoma radiosum; Eo, Etheostoma okaloosae; Es, Etheostoma spectabile; Ejs, Etheostoma jessiae; En, Etheostoma nigrum; Ez, Etheostoma zonale; Et, Etheostoma tallapoosae.
Codons under positive selection were identified in seven of the thirteen mitochondrial protein coding genes by at least one of the methods utilized. Specifically, as shown in Table 1, the MEME method identified two codons in COX1, two codons in CYTB, three codons in ND2, and one codon each in ND3 and in ND5. The FEL method identified one codon in COX1, CYTB and ND3. The codons identified as being under positive selection by the FEL method were also identified as being under positive selection by MEME method. Specifically, codon 489 in COX1, codon 96 in CYTB and codon 9 in ND3. As shown in Table 2, the Selecton method identified one codon in ATP6, one codon in COX3, one codon in CYTB and two codons in ND5. While codons under positive selection were identified in ND5 by MEME and Selecton, these methods did not identify the same codons. Specifically, codon 32 was identified in ND5 by MEME and codons 479 and 573 were identified by Selecton. Only codon 96 in CYTB was identified as being under positive selection by all three methods.
Default significance cutoff value of p<0.1 was used.
| MEME | FEL | |||
|---|---|---|---|---|
| Gene | Site | p value | Site | p value |
| COX1 | 3 | 0.04 | - | - |
| 489 | 0.06 | 489 | 0.04 | |
| CYTB | 82 | 0.09 | - | - |
| 96 | 0.02 | 96 | 0.05 | |
| ND2 | 119 | 0.02 | - | - |
| 327 | 0.06 | - | - | |
| 339 | 0.05 | - | - | |
| ND3 | 9 | 0.06 | 9 | 0.05 |
| ND5 | 32 | 0.08 | - | - |
Ka/Ks ratio indicates the ratio of nonsynonymous/synonymous mutation rates, CI is the confidence interval of the Ka/Ks ratio defined by the 5th and 95th percentiles of the posterior distribution inferred for the position. The p value is the significance of the likelihood ratio test between the M8 model (selection allowed) vs. M8a model (selection not allowed) of the gene.
As shown in Table 3, The changes in codon 96 of CYTB are conservative substitutions of nonpolar amino acids Methionine and Leucine. Similarly, codon 82 of CYTB, codons 119 and 327 of ND2, codon 9 of ND3 and codons 479 and 573 of ND5 also involve substitutions of only nonpolar amino acids. Codon 32 of ND5 involves the conservative substitution of basic amino acids where lysine is present in the Percina species while Arginine is present in the Etheostoma species. While these conservative substitutions were identified as sites under positive selection by the statistical analyses utilized, it is possible that these sites may simply be under relaxed selective stringency. The remaining sites involve substitutions of nonpolar amino acids and polar amino acids threonine and serine (Table 3). These substitutions may have a greater effect upon the structure/function of the relevant OXPHOS complexes. However, the only way to be certain that any of these substitutions identified as being under positive selection are physiologically meaningful, will be to assess the physiochemical functions of these complexes directly.
Pm, Percina macrolepida; Pc, Percina crypta; Ecw, Etheostoma chuckwachatte; Ec, Etheostoma caeruleum; Er, Etheostoma radiosum; Eo, Etheostoma okaloosae; Es, Etheostoma spectabile; Ejs, Etheostoma jessiae; En, Etheostoma nigrum; Ez, Etheostoma zonale; Et, Etheostoma tallapoosae.
While the Bayesian estimates of the Ka/Ks ratio of the sites identified by Selecton are greater than 1, the lower bounds of the confidence intervals defined by the 5th and 95th percentiles of the posterior distribution inferred for these sites are less than 1. Therefore, the reliability of positive selection of these sites identified by Selecton is not very strong. However, positive selection of the four proteins identified by Selecton has been found to be significant (p = 0.001) by the likelihood ratio test of log-likelihood for model M8 (allowing positive selection) versus model M8a (not allowing positive selection).
These results indicate that it is likely that the evolution of various darter lineages included positive selection of at least some of the mitochondrially encoded OXPHOS machinery variants. Presumably, this positive selection would have been driven by adaptation to specific environmental factors. As an example of mitochondrial adaptation to environmental factors, Ma et al. (2015) found that adaptation of Chinese glyptosternoid fishes to high-elevation of the Tibetan Plateau is correlated to signals of positive selection of the Cox1 gene. That adaptation to hypoxia is correlated to physiological adaptation of the OXPHOS machinery has been demonstrated by O2 binding kinetics of cytochrome c oxidase (COX) in hypoxia tolerant vs. intolerant sculpin species (Lau et al., 2017). In this sculpin study, several nonsynonymous substitutions in COX3 were identified by in silico analysis as candidates for explaining the adaptive variation of COX O2 binding. That mitochondrial haplotype variants are subject to selection by environmental factors has been demonstrated experimentally in Drosophila. When a mixed population of “cold adapted” haplogroups and “warm adapted” haplogroups were maintained for a number of generations at differing temperatures, the frequency of the “cold adapted” haplogroup increased at the cooler temperature and decreased at the warmer temperature (Lajbner et al., 2018).
Given the results indicating positive mitochondrial gene selection in this sampling of darter species, it would seem that a more expansive analysis of the evolution of mitochondrial protein coding genes in the approximately 200 extant darter species (Near et al., 2011) may be warranted to identify lineage specific adaptations and, potentially, their correlations to relevant life history traits once those putative adaptations are verified to have altered the physiochemical properties of the OXPHOS machinery.
All mitogenome sequences are available in GenBank.
Percina macrolepida: accession number DQ536430, https://identifiers.org/ncbiprotein:DQ536430.
Percina crypta: accession number KY965073, https://identifiers.org/ncbiprotein:KY965073.
Etheostoma chuckwachatte: accession number KY965071, https://identifiers.org/ncbiprotein:KY965071.
Etheostoma caeruleum: accession number KY660678, https://identifiers.org/ncbiprotein:KY660678.
Etheostoma radiosum: accession number AY341348, https://identifiers.org/ncbiprotein:AY341348.
Etheostoma okaloosae: accession number KY747492, https://identifiers.org/ncbiprotein:KY747492.
Etheostoma spectabile: accession number MK243404, https://identifiers.org/ncbiprotein:MK243404.
Etheostoma jessiae: accession number KY965072, https://identifiers.org/ncbiprotein:KY965072.
Etheostoma nigrum: accession number KT289926, https://identifiers.org/ncbiprotein:KT289926.
Etheostoma zonale: accession number AP005994, https://identifiers.org/ncbiprotein:AP005994.
Etheostoma tallapoosae: accession number KY952221, https://identifiers.org/ncbiprotein:KY952221.
Special thanks to Rachel Moran for access to the E. spectabile mitogenome sequence, to Frank Fontanella for guidance in the use of Mr. Bayes software, and the Warm Springs Fish Technology Center for darter specimens.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Yang Z: PAML 4: phylogenetic analysis by maximum likelihood.Mol Biol Evol. 2007; 24 (8): 1586-91 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: evolutionary genomics
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: evolutionary genomics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
References
1. da Fonseca RR, Johnson WE, O'Brien SJ, Ramos MJ, et al.: The adaptive evolution of the mammalian mitochondrial genome.BMC Genomics. 2008; 9: 119 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: evolutionary genomics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 18 Oct 19 |
read | read |
|
Version 1 14 Apr 19 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)