Keywords
Multiple sclerosis, fatigue, cognitive behavioral therapy, combined therapy, fatigue in MS.
Multiple sclerosis, fatigue, cognitive behavioral therapy, combined therapy, fatigue in MS.
“The idea that the brain can change its own structure and function through thought and activity is, I believe, the most important alteration in our view of the brain since we sketched out its basic anatomy and the workings of its basic component, the neuron.” – Norman Doidge.
Fatigue is a major symptom of multiple sclerosis (MS), which can lead to the difficulty in the carrying out the daily errands and lowers the quality of life; it is prevalent in 80% of patients and hinders the quality of life in nearly 70%1. Fatigue is disabling as it causes problems in daily life necessitating the need for a caregiver, causes embarrassment at workplaces where timebound work is, employment issues that can lead to premature retirement1. Drugs used to treat MS are categorized as oral drugs, injectables, and infusions. Oral drugs include fingolimod, dimethyl fumarate, teriflunomide, and cladribine; injectables include INFβ1a/1b, daclizumab, and glatiramer acetate; infusions include natalizumab, alemtuzumab, and ocrelizumab2. Even upon arrival of new efficacious drugs which can halt the progression of the disease, fatigue remains the most troublesome symptom of patients, giving rise to forms of alternate treatment. This is a systematic review concerning how well pharmacological and non-pharmacological interventions influence fatigue levels in MS patients when compared to healthy adults.
MS is a chronic neurodegenerative disease characterized by disseminated plaque like sclerotic lesions distributed in space and time. They are seen in both grey and white matter of CNS. MS is affecting 2,000,000 people worldwide and 400,000 people in the United States per year. The annual economic burden of the disease in the United States is approximately 10 billion dollars per year1. The 2015 statistics revealed MS disability-adjusted life year (DALY) count was 1234 (1033 to 1437) per 100,000 population, increase in DALY since 1990 to 2015 was 42.4% (31.8 to 57.3%) and age-standardized rate per 100 000 is 17 (14 to 20) per 100 0003. The epidemiological basis of MS is based on genetic and environmental risk factors4. Although we do not have the most recent data on widespread MS investigation, it is estimated that the numbers can be alarmingly higher than the previous records.
Distribution of disease burden according to a survey in 2013 is shown in Figure 1.
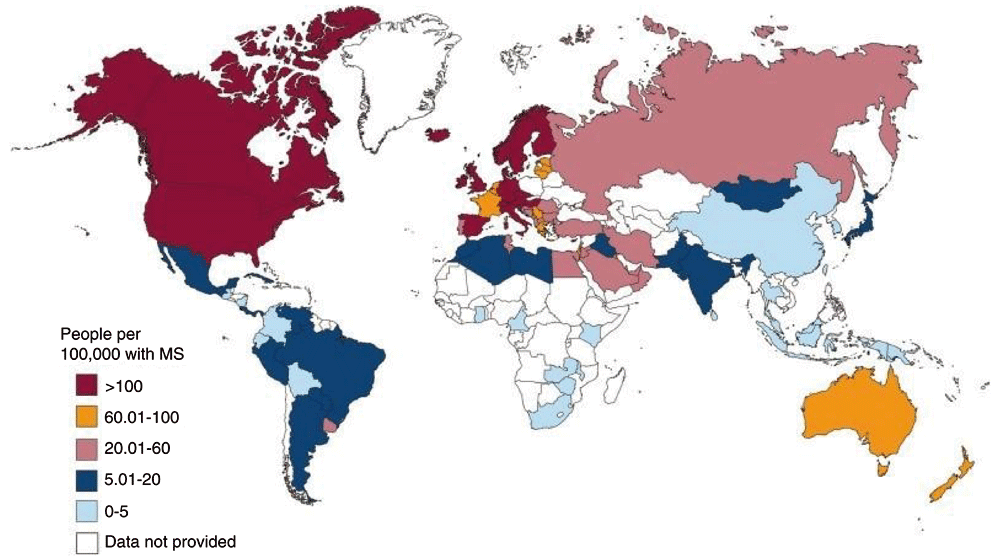
This shows that the disease has a high prevalence in cold countries especially The United States of America and Canada. ©MSIF 2013; reproduced with permission.
MS is characterised by autoreactive T cells like CD4+T cells in the perivascular space and CD8+T cells invading neural parenchyma causing damage to the myelin. Acute sclerosing plaques are due to astrocyte and microglial activation. Microglia clear the dysfunctional synapses that exhibit classical complement proteins C1q and C3. This clearing process can be pathologic if aberrant activation of astrocytes occurs, causing increased complement expression in synapses, resulting in increased degeneration. Neuronal changes, like ballooning of the cell and eccentric nucleus with increased amounts of phosphorylated neurofilaments in the grey matter, are signs of anterograde or retrograde degeneration which could be after effects of axonal disruption in white matter1. The oligodendrocyte precursor cells comprise 5% of CNS cells; they express a proteoglycan called NG2 and can differentiate into mature oligodendrocyte. They also participate in immune reactions by responding to inflammatory cytokines hence limiting our strategy to promote the differentiation of precursor cells to mature oligodendrocytes1. The genome-wide differences present in DNA methylation dictate the susceptibility of damage to oligodendrocytes5. Neuroinflammatory mediators such as INF gamma, TNFα and ILβ promote synaptopathy, demyelination and axonal loss5. This implies that if the inflammatory milieu is stopped, hence the subsequent progression of the disease.
There are four types of MS, relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), primary progressive MS (PPMS) and primary relapsing MS (PRMS). Initially, the disease starts as RRMS and then progresses to SPMS. The disease occurs most commonly in those aged 20–50 years. It occurs more commonly in females than in males, as seen in other autoimmune conditions. The prognosis of the disease depends on the age of presentation and number of exacerbations or relapses of the disease since the initial presentation6. Actively demyelinating lesions in the background of inflammation causing blood-brain barrier dysfunction as seen in RRMS7. Biomarkers of the disease include fetuin-A, nitric oxide synthase and osteopontin8. Symptoms of MS include fatigue, visual problems, cognitive problem, dizziness, gait problem, sensory symptoms, sleep and sexual dysfunction9,10.
The review describes fatigue treatment in MS using pharmacotherapy, exercise therapy and behavioral therapy in the last five years and their efficacy in treatment.
This review was conducted according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement, using the methodology described in Cochrane Handbook for Systematic review of interventions.
The following electronic databases were searched for articles published from the database on September 30, 2018: EBSCO, PubMed, SCIENCE DIRECT and Scopus databases were searched from January 1, 2013, to September 30, 2018. The search strategy included following words “Fatigue and Multiple sclerosis” OR “multiple sclerosis” OR “exercise in MS” OR “pharmacotherapy in MS” OR “Cognitive behavioural therapy and MS”.
All abstracts identified by this search were independently screened by title and abstract by S.K. and T.S. Duplicates were removed by screening based on title of the article and author name. All relevant full-text articles were evaluated for eligibility against the inclusion criteria. Any dispute which arose was solved by mutual consensus. As the scope of the article was limited to systematic review, additional analysis such as sub-group analysis and meta-regression was not done.
The data was extracted independently by two authors S.K and T.S. We collected data from the included randomized controlled trials (RCTs) regarding characteristics of patients, baseline data, expanded disability status scale scores, duration of disease and treatment and outcomes in the study. The changes in fatigue according to different scales was noted in outcomes. We also collected data from systematic reviews in the form of the population included, intervention carried out, comparatives and outcomes of the review with their analytical results on a data extraction sheet. The data was compared and reported while scripting of the discussion. The data of systematic reviews has been exposed to quality analysis using AMSTAR grading shown in Table 1.
| STUDY | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | SCORE (On 11) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. Yang et al.11 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 8 |
| P. Miller and A soundly12 | No | Yes | Yes | No | Yes | Yes | Yes | No | No | No | No | 5 |
| M. Pearson et al.13 | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 8 |
| L.E. van den Akker et al.14 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 8 |
| A.E. Latimer-Cheung et al.15 | No | Yes | Yes | No | Yes | Yes | No | Yes | No | No | No | 5 |
| Fary Khan, Bhasker Amatya16 | UA | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | 6 |
| E. Taylor, R.E. Taylor-Piliae17 | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | 7 |
| Pagnini et al.12 | Yes | No | Yes | No | No | Yes | No | No | No | No | Yes | 4 |
| Phyo et al.1 | UA | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Heine M et al.18 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
| M.Asano, M.L. Finlayson19 | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| H Cramer et al.20 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | 9 |
| Wendebourg et al.21 | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
The articles included in the study are open access and not restricted by region or language. The selection included Randomized controlled trials, observational studies, and systematic reviews. We also included studies which has patients with clinically diagnosed MS and patients >18 years old with fatigue as their presenting complaint. We included studies which reported on patients with both primary and secondary MS. We excluded articles about neuroplasticity in diseases other than MS22–29. We excluded articles which focussed on non-motor aspects of MS or where experimental studies30–42 opinion article43–45, updates46 Letters47 study protocols48 and extended abstracts49. A list of excluded studies is available as Extended data50.
Included studies were independently rated by S.K. and T.S. using the Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. The rating process followed the description in the Cochrane Handbook for Systematic Review of Interventions (part 2:8.5.1) using RevMan version 5.1. Any disagreements during the process was solved by mutual discussions.
The quality of the identified studies was appraised using AMSTAR guidelines51.
We identified 1343 articles from the database search using Scopus, Science Direct, EBSCO and Pub med with no additional articles from other sources (Figure 2). We found 1203 articles to be remaining after removal of duplicates. We excluded 1131 publications based on title and abstract and date of publication. We had 72 full-text articles assessed for eligibility of which 42 articles were excluded among which we excluded articles which related to cognitive changes52–65. We included 10 RCT, 7 observational reviews and 13 systematic reviews1,11–21,66 for the study. A flow diagram is shown in Figure 2.
The study characteristics and summary of systematic reviews is elaborated in Table 2. The study characteristics and summary of RCT is presented in Table 3. The study characteristics and summary of observational studies are depicted in Table 4.
| Study-place- year-design | Population | Intervention | Comparatives | Appraisal | Outcome | No. studies |
|---|---|---|---|---|---|---|
| T. Yang et al. China 201711 | PwMS N=723 F=67.52% | Amantadine Vs n-acetyl carnitine Modafinil | Placebo | Jaded scale Cochrane risk of bias tool | Amantadine proved effective in treating fatigue in MS. L-carnitine was proposed to have similar effect as amantadine. | 11 RCT from 5 databases |
| P. Miller and A Soundy UK 201712 | PwMS N=17469 M-17.8% F-31.7% Rest not known | Amantadine Prokarin, Pemoline Carnitine, Modafinil Vs CBT mindfulness | Reviews including education (active control) No intervention (inactive control) | Amstar grading (Avg=6.5) | Modafinil proved to be beneficial. Pemoline and Carnitine did prove to be beneficial. Combination of physical and cognitive strategy proved beneficial. | 24 Reviews with systematic quantitative RCT From 6 databases |
| Pearson et al. Australia 201513 | PwMS N=655 M-169 F-463 | aerobic endurance training. resistance training aquatics yoga | No exercise | Cochrane RoB tool | pwMS with exercise therapy had reduction in walking time in 10mWT of 1.76 sec. they also showed improvement in walking endurance(6m WT and 2m WT) (P< 0.001) | 13 RCT From 5 databases |
| L.E. van den Akker et al. Netherlands 201614 | PwMS N=520 M-100 F-420 | CBT | Relaxation telephone delivered education and local care | Cochrane RoB tool | Overall CBT had positive short-term effect on fatigue. The long-term effect of CBT based treatment was described in 3 studies. | 6 RCT 9 databases |
| A.E. Latimer- Cheung et al. Canada 201315 | PwMS N=1338* | Aerobic training, resistance training, combined both. | No intervention | PEDro score for RCT Downs and Black scale for non RCT | Exercise done twice a week with moderate intensity increases aerobic capacity with muscle power. It may enhance mobility, fatigue, and health-related QoL. | 23 RTC 31 NON-RTC 7databases |
| Fary Khan, Bhasker Amatya Australia 201716 | PwMS N=16602* | Multiple interventions | No intervention | AMSTAR | Physical therapy for enhanced activity and participation while educational programs reduced fatigue (strong evidence). Multidisciplinary rehabilitation had moderate evidence. Limited for psychological and symptom management programs (fatigue, spasticity). | 15 Cochrane review 24 OTHER REVIEW 5 databases |
| E. Taylor, R.E. Taylor-Piliae USA 201717 | PwMS N=193* | Tai chi | Tai chi group vs non-tai chi or control group | An established tool with 16 study elements | One study proved enhanced cognition and psychosocial fatigue scores (p <0.05). One study reported worsening of fatigue in controls (p < 0.05).Rest revealed no significance | 3 RCT 5 Quasi- experimental studies 13 databases |
| Pagnini et al. Italy 201412 | PwMS N= 5705* | CBT and other psychological treatments | Usual care | QUOROM statements | Fatigue improved following relaxation training, meditation, stress management, and coping. | 22 RCT 4databases |
| Phyo et al. Australia 20181 | PwMS N= 1249* | Psychological interventions CBT | comparators were non-active/active controls (relaxation or psychotherapy) | EPHPP Hamilton Tool | 1. CBT decreased levels of fatigue w.r.t non-active controls (P= 0.07) and with active controls (P = 0.77). 2. Relaxation (P = 0.37) and mindfulness interventions (P= 0.59) decreased fatigue levels compared to non-active control | 20 14 RCT 6- others From 4 databases |
| Heine M et al. Netherlands 201518 | PwMS N= 3206* | Exercise therapy alone vs endurance training vs mixed training vs others | No exercise group two exercise therapies | Cochrane RoB tool | 1. Exercise therapy improved fatigue levels (P < 0.01) and so the others 2. Endurance exercise (P < 0.01) 3. Mixed exercise (P < 0.01) 4. Other exercise (P < 0.01) | 72 RCT 8 databases |
| Asano, Finlayson Canada 201419 | PwMS N= 1499* | Pharmacological Exercise Education | Non-pharmacologic Non-exercise Non-education | Cochrane RoB tool | Rehabilitation- exercise and education have a strong effect in decreasing the impact or severity of fatigue compared to the fatigue medications prescribed very often like Amantadine and Modafinil. Rehabilitation could be the initial treatment of choice contrary to ongoing standards. | 25 RCT 4 databases |
| Holger Cramer et al. Germany 201420 | PwMS N= 670* | Yoga | Usual care, exercise non-pharmacological | Cochrane RoB tool | 1. Yoga had short term effect on fatigue (p = 0.04) 2. No evidence found yoga to be better than exercise (p = 0.83) | 7RCT 7 databases |
| Wendebourg et al. Germany 201721 | PwMS N=1021* | CBT | Non-CBT approaches(education) | Cochrane RoB tool | CBT based treatment approach has a positive effect on fatigue levels.a need for multidimensional treatment emphasized. | 10 RCT 2 databases |
| First author | Crossover design? Randomized/ analyzed (n) | Age in years at baseline, mean (SD) | Characteristics of eligible participants | Treatment groups (n) | EDSS scores, median IQR(SD) | Duration of disease IN YEARS (SD) | Duration of treatment | Summary of findings |
|---|---|---|---|---|---|---|---|---|
| Susan Coote69 | No; 92/65 | 43.3 (9.9) | 1. Physician- confirmed MS cases 2.EDSS score of 0-3 3.sedentary life style | Exercise + social cognitive therapy (SCT) (33) vs exercise + education (32) | 3.3(0.7) | 6.85 (5.9) | 36 weeks | Both groups showed improvements in post- intervention in fatigue levels in PwMS at the end of 36 weeks. This showed a positive effect of behavioral therapy with exercise. |
| Sara Hayes73 | No; 92/65 | 42.6 (9.6) | 1. Physician- confirmed MS cases 2.EDSS score of 0-3 3.sedentary life style | Exercise + SCT (33) vs exercise + education (32) | 3.3(0.7) | 6.85 (5.9) | 36 weeks | ITT analysis showed no difference between the two groups of study. A secondary analysis showed a significant treatment effect favoring the intervention group (p=0.04). |
| Martin Heine71 | No; 89/89 | 45.8 (9.7) | 1. Age ≥18 and ≤70 years ambulant patients 2. expanded disability status scale (EDSS) ≤6.0. 3.no signs of an MS exacerbation or corticosteroid treatment <3months. | Aerobic exercise (43) vs consultation with MS nurse (46) | 3.0 (2.0–3.6) | 8.0 (2.0–15.3) | 16 weeks 12 months follow up. | A short-lived post-intervention effect measured on CIS20r fatigue subscale was seen which did not sustained in follow up period. |
| Jonathan Calkwood67 | No; 1053/1053 | 44.4 to 47.5 | 1. 18–65 years 2. EDSS =0-5.5 3.All have RRMS 4.A single IDMT (except natalizumab) continuously for at least 6 months | 1.GA to fingolimod( n=262) 2.remaining on GA(n=74) 3.IM IFN beta-1a to fingolimod (n=205) 4.continuing IM IFN beta-1a (n=48) 5.SC IFN beta-1a to fingolimod (n=196) 6.continuing on SC IFN beta-1a (n=58) 7.IFN beta-1b to fingolimod (n=125) 8.continuing IFN beta-1b (n=139) | 1)2.5 (1.33) 2)2.4 (1.35) 3)2.5 (1.26) 4)2.4 (1.27) 5)2.4 (1.35) 6)2.3 (1.38) 7)2.4 (1.37) 8)2.5 (1.39) | 13.1 (8.91) 12.2 (9.36) 12.0 (7.9) 11.5 (7.87) 11.1 (7.89) 11.4 (8.04) 12.2 (8.64) 12.3 (6.78) | 6 months | A change to fingolimod from GA, IM IFN beta 1a, SC IFN beta 1 a, IFN beta 1b showed significant changes in TSQM global satisfaction scores. (P <0.001). Remarkable reduction in fatigue in those who switched to fingolimod from SC IFN beta 1 a, IFN beta 1b except those remaining on GA and in IFN beta 1a. |
| Ari J Green74 | Yes; 50/50 | 40.1(10.3) | 1. Stable RRMS patients 2. Disease duration ≤15 years 3. Patients had a demyelinating injury in the visual pathway | 1.Group 1 received 90-day clemastine fumarate followed by 60-day placebo. 2. Group 2 received 90-day placebo followed by 60-day clematine fumarate. There was no washout period | 2.15(1.1) | 4.3(4) | 150 days | Greater improvement in latency in group 2 than in group1. Reduction in latency delay by 1.7msec/eye (p=0.0048) latency reduction was a clinical sign of oligodendrocyte precursor differentiation and re-myelination. Worsening of fatigue was noted on the MAF scale. |
| Arno Kerling75 | No; 60/60 | 42.3 ± 9.0(CWG) 45.6 ± 11.4 (EWG) with a mean of 43.9(10.2) | 1.Maximum value of 6 on EDSS. 2. Adult age (18–65 years) 3. Clinical neurologist- confirmed cases | Combined work out group (30) Vs Endurance work out group (30) | 2.85(1.2) | not mentioned | 3 months | Combined workout group (CWG) and endurance work out group engaged in aerobic training exercise had improvement in aerobic capacity and maximum force. Both groups showed improved fatigue levels. |
| Alexander Tallner76 | No; 126/108 | 40.8 (9.9) | 1.EDSS score of less than or equal to 4.0 2. At least 4 weeks of clinical stability | Internet based e-training (59) vs wait list control group (67) | 2.7 (0.8) | 9.5 (8.2) | 6 months | No significant difference was found between the two groups(including fatigue) except peak expiratory flow (p = 0.01) |
| Fred D. Lublin77 | No; 16/16 | 48.0 (36–58) | 1.Age between 18 and 65 years (both RRMS and SPMS) 2. Disease duration of at least 2 years 3.Evidence of active disease 4. Cardiac, pulmonary, renal and pulmonary function should be normal was required. | 1 unit of PDA-0001(6) vs 4 units of PDA-0001(6) vs placebo (4) | 4.8 (1.5 –6.5) | 8.5 (1.0–31.8) | 6 months with 6 months follow up | 1. It was found safe to infuse mesenchymal like cells to patients with MS 2. No increments in EDSS score more than 0.5. 3.No worsening of MS noted. |
| Marc B. Rietberg72 | No; 48/44 | 46(9.25) | (1) ≥18 years, ambulatory. (2) Clinically diagnosed MS (3) Should have chronic fatigue fulfilling the MSCCPG definition | Multidisciplinary Rehabilitation (23) vs nurse consultations (25) | 3.5(2.5) median (IQR) | 7.5(6.35) | 24 weeks | Within-group effects were found to be insignificant for both groups w.r.t the primary (0.57≤p≤0.97) and secondary (0.11≤p≤0.92) outcome measures from baseline to 12 (P = 0.39) or 24 weeks (P = 0.14) |
| S.Thomas68 | No; 164/131 | 49.05(9.65) | 1.Clincally diagnosed MS. (2) Fatigue with FSS score >4 and (3) Ambulatory | FACETS plus CLP (84) vs CLP (80) | Not available | Different duration from < 1 year to >16 years | 1 year | Statistically significant results were found in the reduction of fatigue severity in the intervention group at the end of 1 and 4 months of following up.no significant difference on MSIS scale at 4 months of follow up. |
| Peter W Thomas70 | No; 164/131 | 49.05(9.65) | 1.Clinically proven MS diagnosis with FSS SCORE > 4 2. Should be ambulatory | FACETS plus CLP (84) vs CLP (80) | Not available | Different duration from < 1 year to >16 years | 1 year | Improvements in self-efficacy and fatigue severity at 4-months of follow-up using FACETS were mostly sustained at 1 year. improvement on MSIS scale was seen which lacked previous assessments. |
| Vanessa Vermöhlen78 | No; 70/67 | 51 (46–55) | 1.MS patients older than 18 years 2.Confirmed MS with spasticity of the lower limbs 3. EDSS score between 4-6.5 | Hippotherapy plus standard care (32) vs standard care alone (38) | 5.4 (0.9) (at inclusion) 21 (31%) = ≤5 46 (69%) = ≥5 | 17.3 (11–23) | 12 weeks | The subgroup with EDSS score of ≥5 (5.1, p = 0.001) showed largest benefit with BBS. Fatigue (p = 0.02) and spasticity (p = 0.03) improved in the intervention group. The mean difference in change between groups was 12.0 (p<0.001) in physical health score and 14.4 (p<0.001) in mental health score of MSQoL-54. |
| Author number of patients (control/ experimental) | Age Of participants Mean ± SD | Characteristics of eligible participants intervention/ treatment | duration of treatment and treatment given | Duration of disease in control and experiment | EDSS scores± SD | Results |
|---|---|---|---|---|---|---|
| Adamczyk-Sowa et al.79 n = 122 (20/102) | 10 ± 11.6 (for controls) 86±10.28 (for RRMS pretreated group), 42±10.06(RRMS INF- beta), 15±7.01 (SP/PP MS Mitoxantrone) (41.90±7.13(RRMS Relapse) | All PwMS diagnosed, according to the McDonald criteria. Melatonin | 90 days melatonin | Control= NA, Pretreated=1.85±1.21, RRMS INFβ=6.27±2. RRMS Mitoxantrone= 20.88±13.65 RRMS Relapse= 6.53±5.13 | Control= 0, RRMS Pretreated= 1.85±095, RRMS INFβ=2.92±1.24, RRMS Mitoxantrone=5.68±1.51, RRMS Relapse =3.96±1.98 | LHP (lipid hydroxy peroxides) and homocysteine concentration was higher in all studied MS groups vs. controls which decreased after melatonin usage. In the RRMS-relapse group levels of homocysteine were significantly higher compared to the RRMS-pre-treated group. The fatigue score was significantly lower in RRMS pre-treated group compared to RRMS-INF-beta and PP/SP MS- mitoxantrone treated patients. |
| Aydin, T.et al.80 n = 40 | 32.83 ± 3.64 | 1. acute exacerbation of MS symptoms. 2. An Ashworth spasticity score over 2 3. EDSS score over 4. | 12 weeks of Calisthenic exercises | 6.97 ±3.15 | < 4.5, Hospital based group = 3.6 ± 1.3, Home based group= 3.4 ± 2.1 | Significant improvements in terms of the BBS, HADS-A and Musi-QoL scores after 12 weeks of home and hospital-based exercises. The HADS-D score improved on the in hospital-based patients only. No significant difference in the FSS score (p < 0.05). |
| Baert, I.et al.81 n = 290 PwMS, 284 completed | 49.7 ± 10.8 | Included subjects had a definite diagnosis of MS, EDSS score ≤6.5 | Single time experiment performed Physical rehabilitation exercises | 11.9 ± 8.1 | 4.8 ± 1.5 | Moderate to severely disabled pwMS performed well on MSWS-12, 2MWT, and 6MWT but not on 25MFWT.(MWT- minute walking test) |
| Burschka, J. M. et al.82 n= 32 PwMS, 15 | TAU= 43.6± 8.0 Tai Chi= 42.6± 9.4 | 1.MS patients (any type) 2. EDSS < 5 3.Relapse free for a month prior study | 6 months Tai chi | TAU*=7.8 ±(6.8) Tai Chi= 6.0±(4.7) | <5 | Balance, coordination and life satisfaction had improved with Tai Chi treatment comparing to TAU. Fatigue and depression were found decreased with Tai Chi treatment. |
| Collett, J et al.83 n = 23, PwMS=14, Control=9 | MS= 52.4 ±8.1, Control= 49.6 ±8.6 | 1) Clinically definite MS, (2) Be the age of 18 years (3) have the adequate mental capacity to consent, (4) Clinically stable (5) Able to use a cycle ergometer. | Single time exercise test performed Multiple intensity exercises | 14.1 SD 9.7 | Not Provided | -Controls proved better on the exercise test (p < 0.05). -PwMS took longer to recover as the intensity of exercise increased (45% at 6 min; 60% at15 min; 90% at 35 min) and correlating with Tympanic temperature. MEParea was significantly depressed in both groups at 45% and 60% (p < 0.001), in the MS group which correlated well to recovery time measured by RPE. RPE= Borg’s ratings of perceived exertion. MEP=motor evoked potential |
| Fernandez-Munoz, J. J. et al.84 n= 108 PwMS | 44 ± 8 | Definite MS according to the modified McDonald criteria. | NA | 12.5±8.0 | 3.4±1.7 | Fatigue score was associated with bodily pain (P<0.01), physical function (P<0.01) and mental health (P<0.01), and with positive association with depression (P<0.01). Depression had negative association with bodily pain (P<0.01) and mental health (P<0.01) Greater the body pain, greater were levels of depression. |
| Soysal Tomruk, M. et al.85 n = 11 PwMS, 12 Control | Median values mentioned in the study MS=52 (35–66) control=50 (38–65) | Age :18 to 65 years, 2≥EDSS score ≤5, | Ten-week | - | 3.5 (2.0–5.0) | Postural control and fatigue levels were significantly worse in PwMS w.r.t healthy controls (p<0.05) but improved sensory interactions were noted (p<0.05) and no effect on postural control (p>0.05). |
A Cochrane review showed exercise therapy to have a significant positive effect on fatigue in RRMS patients [standard mean deviation (SMD) -0.53, 95% confidence interval (CI) -0.73 to -0.33; P-value <0.01] but there was significant heterogeneity [I2>58%] among the trails compared. A few studies showed exercise improved walking speed with 10-minute walking test showing mean difference [MD] reduction in walking time of 1.76 s; [95% (CI), 2.47 to 1.06; P<0.001]66. Another study comes in support of the use of exercise which shows that pooled Effect size was 0.57 (95%CI: 0.10–1.04, P = 0.02)19. These findings suggest that exercise can help to reduce fatigue in MS patients. A study by Taylor et al. mentions a study showing exercise worsening fatigue in MS (P<0.05)17.
Amantadine2,11 is anti-parkinsonian medication that gives an inconsistent improvement in 20–40% of patients over the short term. Yang et al. showed that amantadine might be the most effective drug for treating MS fatigue: SMD and CI were −1.09 [−1.30 to −0.87], and the z-score was 9.75 [P < 0.00001]; however, there was a high variation in number size of patients, causing heterogeneity to be 91%11. The two most effective drugs in treatment are natalizumab and alemtuzumab, but they cause progressive multifocal leukoencephalopathy (PML) due to John Cunningham virus and autoimmune diseases of thyroid along with thrombocytopenic purpura with immune glomerulonephritis respectively. A 6-month study in 2016, ECTRIMS showed no increase in mortality. Ocrelizumab, the first drug effective to slow down PPMS and which targets B cells in RRMS and PPMS, is in a phase 3 trial2. A counter drug in SPMS is still to be discovered as IFNβ1b has not shown efficacy in American SPMS trials. Hence trials should be performed with combination therapy including ocrelizumab and IFNβ1b to counter SPMS, which has a poor prognosis2.
Cognitive behavioural therapy [CBT] can help reducing fatigue in MS (pooled SMD = −0.71, 95% CI: −1.05 to −0.37, P = 0.77) as compared to active controls1. Supporting studies also show a positive effect [SMD] = −0.47;95% [CI] = −0.88; −0.06; I2 = 73%]. A long-term positive effect of CBT [SMD = −0.30; CI −0.51; −0.08; I2 = 0%] is also shown but had limited number of studies14. Thus, CBT shows a positive effect on fatigue in MS. Practices like yoga show some effect compared to usual care [SMD = 20.52; 95% CI = 21.02 to 20.02; p = 0.04] but fail to prove better than exercise therapy [SMD = 0.03; 95% CI = 20.24 to 0.30; p = 0.83]23.
Trials based on pharmacotherapy have shown that a change to new drugs like oral fingolimod was beneficial to many patients for fatigue in MS as shown by EPOC trial. The TSQM Global satisfaction scores were superior after the switch from intravenous disease-modifying therapy iDMT to oral fingolimod [p<0.001]67. Aerobic training exercises were delivered in ambulatory MS patient which showed improvements. This view was supported by the TREFAMS-AT trial (p<0.014). The non-fatigue related outcomes such anxiety, depression, and cognition showed improvement in the certain trials, which explains the dynamic connections with fatigue as a symptom67,68.
Exercise therapy is a potential treatment modality, and when combined with education therapy it can cause behavior modification in many patients. This view was supported by the STEP IT UP and FACETS trial. It was able to prove that mobility was increased in intervention groups through the intervention time was relatively short (10 weeks)68–70.
The chronicity of symptoms in MS has a tremendous impact on the probability to show improvement to any therapy. It will be difficult to expect a positive change in a patient who has suffered chronic fatigue when compared to fatigue of new onset in MS patients. A study showed that multi-disciplinary rehabilitation on chronic fatigue patients was not effective in bringing the fatigue levels to a significant low that could be appreciated subjectively71,72.
All criteria were judged as low, high or unclear risk of bias. In summary, most of the studies had a low risk of bias. The risk of bias graph is show in Figure 3 and Figure 4. Calkwood et al.67, had high risk of bias as it lacks random sequence generation and allocation concealment. Calkwood et al.67, Thomas et al.70, failed to fulfil blinding of participants and outcomes in their respective studies, which were thus prone to performance and detection bias. It was unclear in a few studies whether allocation concealment and blinding of participants was carried out in studies like Heine et al.71 and Rietberg et al.72.
As a result of heterogeneity among studies due to different study designs taken into consideration and a smaller number of participants in various studies owing to loss of follow up and the pathogenicity of the disease, a meta-analysis was not carried out.
The primary outcomes in most of the trials used MSIS, FSS and CIS-20R scales69–71. MSIS is a subjective scale based on a patient experiencing fatigue. CIS-20R subscale measures fatigue severity. FSS measures the impact of fatigue on normal functioning. The changes measured on any scale should be accompanied by a change in FSS scale to make it clinically meaningful to adopt as a standard measure for generalizability. Not every severely fatigued patient (in most cases of advanced MS) will give expected results on standard exercising protocol. It is an arguable viewpoint leading to reverse causality whether exercise therapy is worsening fatigue levels in MS patients as supported by a systematic review by Taylor et al.17.
Considering the therapeutic interventions for MS-related fatigue, a variety of exercise methods (pilates85, calisthenics80, Tai Chi82 and aerobic) of exercises have gained attention. Numerous studies have shed light on the efficiency of exercise for the PwMS, almost all studies designated the exercise as a remarkable factor in improving the fatigue and its related distress in MS84. Certain observational studies have been conducted to find out if the cause of fatigue in PwMS is the physical activity instead of neural demyelination and lack of neuroplasticity. One cross-sectional study ruled out the possibility of fatigue associated with the physical activity instead they found a strong association between the mental health and fatigue84.
Fatigue in MS can be due to depression, which intercedes the association between neuronal issues and physical conditions84. Pharmaceutical interventions like melatonin supplementation have been effective to treat the fatigue related to MS79. Melatonin can act as anti-inflammatory and immunomodulatory drug that does not cross the blood-brain barrier. The anti-oxidative effect can be used to treat MS patients as they have high oxidative stress owing to elevated levels of plasma lipid peroxide and activated nitrosative-oxidative pathways79.
An observational retrospective study showed that switching from interferon-β to glatiramer improved work productivity, activity impairment and health-related quality of life [HRQoL] and fatigue. Transcranial magnetic stimulation [TMS] is an innovative way to record neurophysiological responses by measuring corticospinal-neuromuscular pathway excitability. The persistent excitation of brain neurons plays an important role in progressive forms of the disease83.
Plasticity is a functional reorganization of neurons carried out through anatomical reorganization and axonal sprouting with synaptogenesis. Physical training is known to induce compensatory plasticity and increases activity-dependent synaptic potentiation. Exercise is known to cause an increase in endocannabinoid signalling86.
The summary of included observational studies can be found in Table 4.
The diversity of pathological phenomena involved in fatigue related to MS is a major concern in understanding and quantifying the role of each causal factor. Our study has found a significant positive effect on fatigue levels of RRMS patients with regular CBT and exercise-based combination therapy. These results were not supported in case of PPMS or SPMS patients due to the aggressive nature of the disease. Emphasizing the clinical significance of combinational therapy which can be prescribed in MS, yet this does not undermine the need for statistical analysis and correlation. Future research should focus on improving the quality of life of progressive forms of MS. Factors responsible for a high drop-out rate should be studied and correlated with morbidity and mortality rates. We believe an amalgamation of sound mental health, physical health, and pharmacological health shall tone down or blunt the effect of fatigue in the daily life of MS patients.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: DIVERSE MECHANISMS AND TREATMENT STRATEGIES TO CONFRONT FATIGUE IN MULTIPLE SCLEROSIS -A SYSTEMATIC REVIEW. https://doi.org/10.17605/OSF.IO/W5DA450
This project contains references for all excluded studies.
Extended data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Open Science Framework: PRISMA checklist for study “Diverse mechanisms and treatment strategies to confront fatigue in multiple sclerosis: A systematic review”. https://doi.org/10.17605/OSF.IO/W5DA4
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
No
References
1. Lublin FD, Reingold SC, Cohen JA, Cutter GR, et al.: Defining the clinical course of multiple sclerosis: the 2013 revisions.Neurology. 2014; 83 (3): 278-86 PubMed Abstract | Publisher Full TextCompeting Interests: Tomas Kalincik served on scientific advisory boards for Roche, Sanofi-Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi-Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Novartis, Biogen, Genzyme-Sanofi, Teva, BioCSL and Merck and received research support from Biogen. None of these disclosures is directly relevant to the presented article or its topic.
Reviewer Expertise: multiple sclerosis, neuroimmunology, biostatistics, clinical outcomes research
Are the rationale for, and objectives of, the Systematic Review clearly stated?
No
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
No
Are the conclusions drawn adequately supported by the results presented in the review?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Methodology and publications in this area including one cited by the authors.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Apr 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)