Keywords
Aldose reductase, Protein unfolding, Folding intermediate, Cooperativity, Tryptophan fluorescence, ANS fluorescence, Thermal denaturation
Aldose reductase, Protein unfolding, Folding intermediate, Cooperativity, Tryptophan fluorescence, ANS fluorescence, Thermal denaturation
We are thankful to the reviewers for their valuable comments. In response to the comments, we have changed the manuscript. The followings are significant changes from the previous version of the manuscript.
1. We have included additional references and have stressed the relevance of the study.
2. Figures have been revised as per suggestion by the reviewer(s).
3. The word 'thermal unfolding’ has been replaced by 'thermal denaturation.'
4. We have gone through the manuscript carefully and have made necessary changes regarding linguistic issues.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
GuHCl, guanidine hydrochloride; TCEP, (tris(2-carboxyethyl)phosphine); ANS, 8-anilino-1-naphthalenesulfonic acid ammonium salt; IPTG, isopropyl β-D-1-thiogalactopyranoside; Trp, tryptophan.
Human aldose reductase (hAR) (EC 1.1.1.21) is an NADPH-dependent oxidoreductase that belongs to the superfamily of aldo-keto reductases1. hAR is the first and rate-limiting enzyme of the polyol pathway and converts glucose to sorbitol2. The polyol pathway is up-regulated under hyperglycemic conditions, and a significant proportion of glucose gets fluxed through this pathway, which leads to the accumulation of sorbitol, consumption of NADPH, and redox imbalance of NADPH/NADP+ ratio. All these factors have been linked with various tissue-based pathologies associated with secondary complications of diabetes mellitus3. Due to its clinical importance from the perspective of developing potent inhibitors to prevent or delay the onset of secondary diabetic complications, hAR has been widely studied in almost every aspect4.
Extensive information is available in the literature about the structure and function of hAR, mainly related to active site of hAR from high-resolution crystal structures with a number of potential inhibitors5, flexibility in the hAR binding site pocket6 and the thermodynamics of closing/opening of the specificity pocket within binding site pocket of hAR7. There is little investigation related to the folding/unfolding mechanism of hAR.
Protein folding is a fundamental process in all living systems. The linear sequence of amino acids encodes the information required for a polypeptide to fold. Despite extensive research and progress made over the years to understand this fundamental process, a complete understanding of protein folding mechanism(s) and the folding code remains elusive8. Various models have been proposed to explain the mechanism underlying protein folding reaction from time to time9. ‘New view folding model’ based on energy landscape theory (ELT) and ‘defined pathways model’ based on the concept of foldons are current alternatives to explain the protein folding mechanism.10.
Among several hallmarks of protein folding are its spontaneity and cooperativity, along with fast reaction timescale. Usually, a small free energy change is required to shift the equilibrium between the folded and unfolded state ensemble. Complex cellular solvation environment plays a vital role in regulating the equilibrium between folded and unfolded ensemble in vivo, which are separated by small free energy barrier (~5 - 10 Kcal mol-1)11.
Under physiological conditions, protein structure fluctuates among different native conformations separated by close free energy barriers12. Since hAR activity leads to sorbitol accumulation, leading to osmotic stress, it seems to function under stress conditions which might perturb its native conformation ensemble. Here we report on thermal denaturation and chemical induced unfolding studies of hAR. Thermal denaturation revealed a simple two-state transition, whereas chemical induced unfolding led us to discover an intermediate state during hAR unfolding.
The hAR cDNA cloned into expression vector pET-15b (Novagen) was a kind gift from Dr. Alberto Podjarny (Department of Integrated Structural Biology, Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS, INSERM, France). The plasmid, coding for a hexahistidine-tagged protein, was expressed into E. coli strain BL21 (DE3) (Novagen). The cells with recombinant plasmid were grown with 100 μM ampicillin at 37°C to an OD600 nm value of 0.7, and protein expression was induced by adding 1 mM IPTG. Cells were grown for a further 3 hours at 37°C. All further operations were carried out at 4°C unless otherwise stated. Cells were centrifuged, resuspended, and lysed by sonication. A Ni-NTA affinity column (GE Healthcare) was used for protein purification. The material used for the stationary phase for the column was Ni-Sepharose, and the flow rate of the column was adjusted to 0.5 ml min-1. Imidazole and other salts were removed by repeated dialysis in 50 mM potassium phosphate, pH-7 buffer containing 50 mM NaCl. Protein concentration was estimated using the molar extinction coefficient and absorbance reading at 280 nm. The histidine tag from recombinant protein was removed by thrombin (4 units of thrombin per mg of recombinant protein at room temperature for 3 hours). The cleaved protein was passed through the Ni-NTA column, and purified protein without tag was collected as flow-through. Enzyme activity was checked as per standard assay13. We analyzed the homogeneity and molecular weight of hAR with and without histidine tags under denaturing conditions on 15% SDS-PAGE. Purified hAR was stored at -20°C for further studies.
Thermal denaturation was carried out at a final concentration of 2.8 µM protein in 50 mM potassium phosphate buffer, pH 7.0, containing 50 mM NaCl and 0.1mM TCEP. The transition between 20–70 °C was followed using a far-UV circular dichroism (CD) signal at 222 nm by using a 0.1 cm path-length cuvette at a sampling rate of 1.0 °C min-1 in a Jasco J-810 spectropolarimeter. After subtracting buffer blank, we have reported the change in ellipticity (millidegree) at 222 nm.
To follow chemical induced unfolding, we prepared samples with 1.4 μM protein concentration in phosphate buffer (described earlier), containing different concentrations of GuHCl/urea. Samples were incubated for 12 hours to reach equilibrium at 25°C, after which no change in signal occurred either in fluorescence or CD spectra. Trp fluorescence (excitation at 295 nm and emission recorded between 300 nm to 400 nm) and ANS fluorescence (excitation at 370 nm and emission recorded between 400 nm to 600 nm) measurements were performed using a Hitachi F-7000 fluorescence spectrophotometer for GuHCl samples and Jasco J-815 spectropolarimeter for urea samples. Far-UV CD measurements were performed using Jasco J-810 spectropolarimeter for GuHCl samples and Jasco J-815 spectropolarimeter for urea samples. Quartz cuvette of 1 cm and 0.5 cm path-length were used for fluorescence and CD measurements, respectively. All measurements were done at 25 °C. We have reported spectra as ellipticity (millidegree) after baseline correction.
We have calculated the value of ΔCp for the unfolding of hAR from the change in accessible surface area (ΔASA) according to Equation 114
ProtSA webserver was used to calculate the change in accessible surface area from native to the unfolded conformation of hAR15.
GraphPad Prism version 7.04 for Windows (GraphPad Software, La Jolla, California) was used for the analysis of thermal denaturation and chemical-induced unfolding data based on two-state and three-state models respectively, as described in following sections.
The Least-square analysis was used to fit the data to Equation 216.
Where An and Au are native and unfolded state baseline intercepts, respectively, and bN and bU are native and unfolded baseline slopes, respectively. ΔHm is enthalpy change at melting temperature (Tg). T is absolute temperature, and R is the gas constant.
Signal for native (YN = An + bN × T) and unfolded baseline (YU = Au + bU × T) for every point in transition region was calculated from Equation 2. If Y is signal for a particular point in transition region, then the fraction of unfolded protein (Fu) at this point is given by Equation 3.
The equilibrium constant (k) was calculated from the relative population of species using Equation 4.
ΔG was calculated as a function of temperature using Equation 5.
The thermal stability curve of hAR was constructed based on Equation 6–817.
Where ΔHT and ΔST are enthalpy change and entropy change, respectively, at temperature T with reference to Tg. Th, Ts and Tg are the temperatures at which ΔH, ΔS, and ΔG are zero, respectively. ΔGs is the stabilization free energy of the native state relative to the unfolded state.
The least-square analysis was used to fit the data to Equation 916.
Where An, Au, and Ai are the native, unfolded, and intermediate baseline intercepts, respectively, and bN, bU, and bI are the native, unfolded, and intermediate baseline slopes, respectively. [D] is denaturant concentration in a molar unit. m(N-I) and m(I-U) are denaturant gradient for native to intermediate and intermediate to unfolded state, respectively. ΔG(N-I) and ΔG(I-U) are stabilization free energy of intermediate state relative to the native and unfolded state, respectively.
Change in ellipticity at 222 nm fitted well based on a two-state model (Figure 1A). This analysis gave values for ΔHg and Tg, which along with the ΔCp value calculated from Equation 1 were used for non-linear regression of the transition region (±5 kJ mol-1) to Equation 8 (Figure 1C). Values of ΔH and ΔS were calculated over an extended range of temperatures by using Equation 7 and Equation 8, respectively (Figure 1D). The thermal stability curve is an extrapolation of transition region, assuming constant ΔCp during the unfolding transition (Figure 1E). The relationship between Ts, Th and ΔG (Ts – Th = ΔGs/ΔCp) is presented in Figure 1F. Thermodynamic parameters obtained from the analysis of thermal denaturation data are listed in Table 1. All raw data are available as Underlying data18.
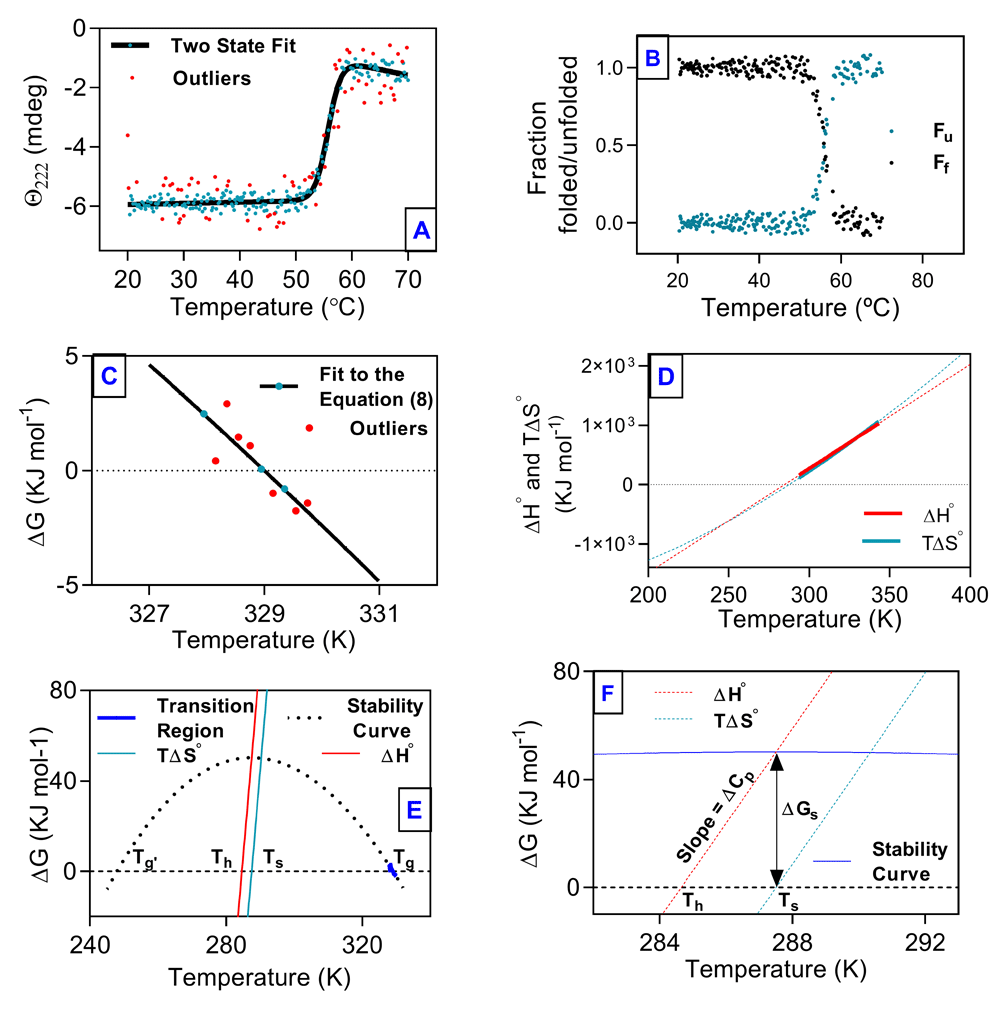
(A) Change in ellipticity at 222 nm plotted as a function of temperature. (B) The fraction of protein folded (black dots) and unfolded (cyan dots) plotted against temperature. (C) The portion of the transition curve used in van't Hoff analysis. (D) Plots of ΔH and ΔS as a function of temperature. (E) Thermal stability curve of hAR. (F) Triangular relationship among Th, Ts, and ∆Gs. The explanation for Tg, Tg’, Th, and Ts is given in the text. Dashed lines are extrapolations.The solid lines represent curves fitted to the unfolding transition; filled symbols represent data points from unfolding experiments; and red symbols represent outliers.
There are six Trp residues in hAR, out of which four are part of the hydrophobic active site pocket in the core of the β-barrel, and two are buried in alpha-helices surrounding the barrel. Their fluorescence provided a global signal of change in the tertiary structure. ANS has been extensively used as a probe for non-native, partially unfolded conformations of the protein. The binding of ANS to hydrophobic regions results in a significant enhancement of ANS fluorescence and a pronounced blue-shift of the λmax19.
Fluorescence emission profiles of hAR equilibrated with different concentrations of denaturants are presented in Figure 2 (Figure 2A and Figure 3A for urea and GuHCl, respectively). A plot of λmax against denaturant concentration indicated a cooperative transition from native to unfolded state (Figure 2B and Figure 3B for urea and GuHCl, respectively). In the case of ANS fluorescence, a significant blue-shift of around 20 nm and 10 nm from the native to the intermediate state was observed for urea and GuHCl, respectively (Figure 2B2 and Figure 3B2 for urea and GuHCl, respectively).

(A1) Trp fluorescence scans and (A2) ANS fluorescence scans for all the samples. (B1) λmax (Trp fluorescence) and (B2) λmax (ANS fluorescence) against [urea]. (C1) Imax (Trp fluorescence) and (C2) Imax (ANS fluorescence) against [urea]. (D1) I295/314 (Trp fluorescence) and (D2) I370/480 (ANS fluorescence) against [urea]. (E1) Trp fluorescence and (E2) ANS fluorescence of samples in native (black), intermediate (cyan), and unfolded state (red). Solid lines represent curves fitted to the unfolding transitions; filled symbols represent data points from unfolding experiments; red symbols represent outliers.

(A1) Trp fluorescence scans and (A2) ANS fluorescence scans. (B1) λmax (Trp fluorescence) and (B2) λmax (ANS fluorescence) against [GuHCl]. (C1) Imax (Trp fluorescence) and (C2) Imax (ANS fluorescence) against [GuHCl]. (D1) I295/314 (Trp fluorescence) and (D2) I370/480 (ANS fluorescence) against [GuHCl]. (E1) Trp fluorescence and (E2) ANS fluorescence of samples in native (black), intermediate (cyan), and unfolded state (red). Solid lines represent curves fitted to the unfolding transitions; filled symbols represent data points from unfolding experiments; red symbols represent outliers.
A plot of Imax against denaturant concentration indicated the presence of an intermediate during unfolding transition (Figure 2C and Figure 3C for urea and GuHCl, respectively). Imax in case of ANS fluorescence fits satisfactorily based on the three-state model (Figure 2C2 and Figure 3C2 for urea and GuHCl, respectively). For both urea and GuHCl induced unfolding, Trp fluorescence emission intensity at 314 nm fits satisfactorily to the three-state model. In the case of ANS fluorescence, both Imax and fluorescence emission intensity at 480 nm fit equally well based on three-state model. Thus, Trp fluorescence emission intensity at 314 nm and ANS fluorescence emission intensity at 480 nm were analyzed based on the three-state model to evaluate the thermodynamic stability of hAR (Figure 2D and Figure 3D for urea and GuHCl, respectively). The thermodynamic parameters obtained from the fittings are listed in Table 1. Trp and ANS fluorescence demonstrate the presence of an intermediate state populated at 3.5-4.0 M and 0.7-2 M urea and GuHCl concentration, respectively, apart from the native and unfolded states (Figure 2E and Figure 3E for urea and GuHCl, respectively).
Unfolding profiles of hAR equilibrated at different denaturant concentrations monitored by far-UV CD are presented in Figure 4A1 and 4A2 for GuHCl and urea, respectively. The thermodynamic stability of hAR was determined based on the three-state model by plotting the change in ellipticity at 219/222 nm as a function of denaturant concentration (Figure 4B1 and 4B2 for GuHCl and urea, respectively). The transition determined by far-UV CD detected intermediate state at similar concentrations of denaturant as interrogated by fluorescence spectroscopy. Far-UV CD profiles clearly distinguish three states (Figure 4C1 and 4C2 for GuHCl and urea, respectively). Thermodynamic parameters derived from far-UV CD data are listed in Table 1.

(A1) far-UV CD scans recorded for GuHCl and (A2) far-UV CD scans recorded for urea for all the samples. (B1) change in ellipticity at 219 nm against [GuHCl] and (B2) change in ellipticity at 221.8 nm against [urea]. (C) CD spectra of samples in native (black), intermediate (cyan), and unfolded state (red) for (C1) GuHCl and (C2) urea, respectively. The solid lines represent curves fitted to the unfolding transitions; filled symbols represent data points from unfolding experiments; red symbols represent outliers in data fitting.
The intermediate state with enhanced ANS fluorescence and significant blue shift of λmax pointed to an intermediate state with ‘molten like’ nature during the chemical-induced unfolding of hAR. Far-UV CD studies strongly suggest that the intermediate state retains significant secondary structure during urea- and GuHCl-induced unfolding.
During chemical induced unfolding, hAR unfolds through an intermediate state, which is absent during thermal denaturation. Moderate concentration of denaturant is known to stabilize native or intermediate state20. The absence of such a stabilizing agent may be the reason that we could not detect the intermediate state during thermal denaturation.
In all three probes used in studying unfolding, the value of ΔG(N- I) obtained is ~30 kJ mol-1 and ~15 kJ mol-1 for urea- and GuHCl-induced unfolding, respectively while a ΔGs of ~70 kJ mol-1 is almost same for both denaturants (Table 1). Thus, while urea seems to stabilize the native state with respect to the intermediate state, GuHCl seems to stabilize the intermediate state with respect to the native state.
Small molecules change the free energy landscape of protein upon binding by selectively stabilizing native or intermediate/unfolded state21. The difference in the value of ΔGs obtained from thermal denaturation and chemical-induced unfolding is ~20 kJ mol-1 (Table 1), which is most likely due to free energy of stabilization and destabilization by urea and GuHCl, respectively.
Values of ΔG(N-I) obtained from the analysis of ANS fluorescence data are 16.48 and 28.61 kJ mol-1 for GuHCl and urea, respectively (Table 1), which indicates that a steep energy barrier does not separate intermediate state from the native state. Values for ΔG(I-U) obtained from ANS fluorescence are 57.26 and 38.6 kJ mol-1 for GuHCl and urea, respectively (Table 1), which indicates that the intermediate state is separated from unfolded state by a high energy barrier. Thus, the intermediate state of hAR is close to its native state, which makes it functionally more relevant. These studies are essential to access the effect of other physiological relevant molecules on hAR stability22. Protein stability of hAR in the cellular environment under hyperglycemia-induced cellular stress conditions has to be studied to aid rational drug design23.
In summary, equilibrium unfolding studies of hAR have led us to discover that hAR unfolds through an intermediate state, which is close to the native state, and might have physiological relevance under hyperglycemic conditions in diabetes.
Figshare: data_f1000_hAR_unfolding.zip. https://doi.org/10.6084/m9.figshare.8001998.v118.
This project contains raw data for thermal denaturation and chemical induced unfolding studies on human aldose reductase.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We acknowledge Dr. Alberto Podjarny (Department of Integrated Structural Biology, Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS, INSERM, France.) for hAR plasmid as a kind gift, Dr. Yogendra Sharma, at CSIR-CCMB, Hyderabad and Chairperson, Department of Biotechnology, Panjab University, for helping us in spectroscopic data collection. Gurprit acknowledges research fellowship from UGC, Govt. Of India (UGC science JRF).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biophysics, biochemistry, cell biology, enzymology, spectroscopy, protein folding,misfolding and aggregation, aggregation diseases, proteostasis, cataract, mRNA decay, deadenylation, translation regulation
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Povarova OI, Kuznetsova IM, Turoverov KK: Differences in the pathways of proteins unfolding induced by urea and guanidine hydrochloride: molten globule state and aggregates.PLoS One. 2010; 5 (11): e15035 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Biophysics, biochemistry, cell biology, enzymology, spectroscopy, protein folding,misfolding and aggregation, aggregation diseases, proteostasis, cataract, mRNA decay, deadenylation, translation regulation
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Povarova OI, Kuznetsova IM, Turoverov KK: Differences in the pathways of proteins unfolding induced by urea and guanidine hydrochloride: molten globule state and aggregates.PLoS One. 2010; 5 (11): e15035 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Protein physics; protein folding; partially folded proteins; folding intermediates; protein misfolding' protein aggregation; conformational diseases; intrinsically disordered proteins
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Pastore A, Martin SR, Temussi PA: Generalized View of Protein Folding: In Medio Stat Virtus.J Am Chem Soc. 2019; 141 (6): 2194-2200 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 05 Nov 19 |
read | read | |
|
Version 1 26 Apr 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)