Keywords
Neurocritical care, EEG, non-convulsive seizures, status epilepticus, Saudi Arabia
Neurocritical care, EEG, non-convulsive seizures, status epilepticus, Saudi Arabia
This version of the article contains more details about the clinical setting in which the data were gathered, including the general guidelines clinicians follow in the ICU to select patients for CEEG and to decide on starting and stopping monitoring. Furthermore, this version has a flow chart summarizing the study data and EEG examples of lateralized periodic discharges and non-convulsive seizures obtained from the data. The results have been scrutinized and additional results about the frequency of seizure etiologies and the frequency of different rhythmic and periodic patterns are now included. The discussion highlights that CEEG is effective in detecting potentially harmful subclinical EEG patterns that are worth treating but when it comes to prognosis and outcome there are uncertainties whether the data provided by CEEG are clinically meaningful. The limitations of the study are clearly stated, including the lack of accurate knowledge of the timing of the EEG in relation to mental status changes, patient outcomes, and sedative use. This is in addition to the practical limitations of the developing CEEG program, the small numbers in some subgroups, and the retrospective study design. The discussion argues that the current data should compel decision-makers in developing health care systems to support the development of ICU CEEG programs with embedded prospective research that helps optimize the pattern of use of CEEG and guide the field toward providing data that are clinically meaningful to patient care in the ICU.
See the author's detailed response to the review by Robert C. Tasker
See the author's detailed response to the review by Peter W Kaplan
See the author's detailed response to the review by Yara Mikhaeil-Demo and Elizabeth E Gerard
Continuous electroencephalography (CEEG), the practice of continuously recording an electroencephalogram and a time-synchronized video of the patient, is commonly utilized to monitor critically ill patients with acute brain injury or altered mental status1. CEEG is instrumental in the diagnosis and management of nonconvulsive seizures (NCS) and status epilepticus, detection of cerebral ischemia, prognostication of outcomes after cardiorespiratory arrest, and evaluation of abnormal movements and altered mental status1. The practice of CEEG monitoring of critically ill patients in the intensive care unit (ICU) has been spreading in Europe and North America over the past decade1,2. Building an effective ICU CEEG program with sufficient quality demands not only adequate EEG equipment but also significant human resources2, including trained electroencephalographers and technologists with enough time to review large amounts of CEEG data.2. While this is available in large tertiary care centers in North America and Europe where the practice of CEEG has developed, it may not be available in developing healthcare systems with constrained resources.
This study reviewed data generated from a CEEG program in the adult ICU at a tertiary healthcare center in Saudi Arabia, aiming to shed light on the real-life utility of CEEG in a developing healthcare system outside North America and Europe.
This is a retrospective review of ICU CEEG findings, as well as mortality status and duration of hospitalization of all patients who underwent CEEG monitoring during a 12-month period from September 2016 to August 2017 at the adult ICU at the King Abdulaziz University Hospital (KAUH) in Jeddah, Saudi Arabia. This is an academic, tertiary-care, 600-bed hospital. Its adult ICU is comprised of 30 beds and is divided into medical and surgical divisions. The average APACHE II (Acute Physiology and Chronic Health Evaluation II) score of medical patients admitted to the ICU ranges between 10–30 on average. The scores are routinely calculated but not recorded in the electronic medical records (EMR). ICU physicians or neurologists request CEEG to search for NCS or patterns on the ictal-interictal continuum when critically ill patients have a disturbance in the level of consciousness that is unexplained by apparent underlying neurological or medical conditions. CEEG is initiated and stopped based on the clinical judgement of the treating teams. Generally, physicians aim to continue monitoring for 24 hours in patients with altered mental status but may allow discontinuation of CEEG for clinical or practical constraints (e.g. EEG machine availability or the need to relocate a patient to conduct an procedure or test). EEGs with a duration that is less than 2 hours were not included in the study as they were considered extended but not long-term studies. An EEG technologist is available during the day time to set up ICU CEEGs. EEG leads are placed using the 10–20 international system of lead placement. CEEGs are digitally recorded, including synchronized video recording of the patient. An epileptologist with fellowship training in CEEG interpretation reviewed the records on daily basis and reported them using the American Clinical Neurophysiology Society (ACNS) ICU EEG consortium proposed nomenclature for ICU EEG reporting, and the Salzburg criteria for non-convulsive status epilepticus3.
Reports of CEEGs performed in the adult ICU during the study period were retrieved from the hospital’s EMR. The author extracted key data, including background demographics, diagnoses, length of hospital stay, mortality status at 60 days after admission, and the presence of rhythmic and periodic patterns (RPPs) or NCS. Frequencies, percentages, means, standard deviation, and Chi square were performed using the IBM SPSS Statistics for Windows, version 20.0.
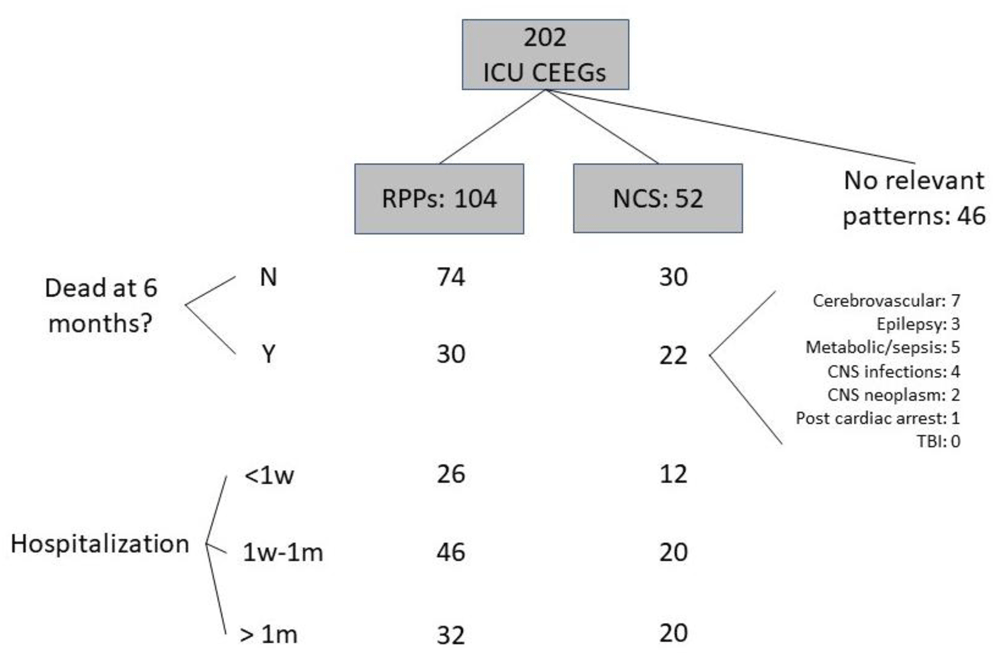
A total of 202 CEEG records fulfilling the criteria were identified; complete, raw figures are available as Underlying data4. There were 116 female patients. The mean age was 53 (standard deviation=21). The primary diagnosis was cerebrovascular disease in 61 patients (30.2%), Epilepsy in 53 patients (26.2%), sepsis or metabolic derangement in 40 patients (19.8%), CNS infection in 24 patients (11.9%), post-cardiac arrests in 10 patients (5%), brain neoplasm in 8 patients (4%), and traumatic brain injury in 6 patients (3%). The duration of CEEG recording varied, with 48 (24%) recorded for 2–6 hours and 154 (76%) recorded for 6–24 hours. Figure 1 shows a flowchart demonstrating the frequencies of CEEG findings in the sample and their distribution over the outcome categories. There were 52 patients with NCS. Among them, 30 were of focal onset (57.7%) and 10 (5%) clearly fulfilled criteria for non-convulsive status epilepticus. There were 120 patients that had clinical seizures prior to CEEG monitoring. Among them, 36 (30%) had NCS on EEG. A total of 138 records showed RPPs, including 26 that had more than one RPP and 34 of the 52 records with NCS. A total of 78 records had only one type of RPP, including 22 (10.9%) with generalized periodic discharges (GPDs), 20 (9.9%) with lateralized periodic discharges (LPDs), 22 (10.9%) with generalized rhythmic delta activity (GRDA), and 14 (6.9%) with lateralized rhythmic delta activity (LRDA). Figure 2 shows EEG examples of LPDs and focal and generalized NCS. Sixty two patients (30.7%) out of the entire sample were deceased at 60 days after admission. This proportion was significantly higher in patients who had NCS than those who didn’t (42% vs 27%, χ2 = 4.4, df=2, p=0.03) (Table 1). The frequency of NCS etiologies in the group that was deceased at 60 days is shown in Figure 1. There was no statistically significant difference in mortality risk between the etiologies. There was no significant difference in the duration of hospital stay between those who had seizures and those who didn’t (p=0.2) (Table 1). Sixty-four patients stayed in the hospital for more than a month (31.7% of the entire sample). This proportion was slightly larger in patients with RPPs (33.3%). The duration of hospital stay was longer for those who had RPPs than those without RPPs (χ2 = 8.02, df=2, p=0.02) but there was no significant relationship between RPPs and mortality at 60 days (χ2 = 1.1, df=2, p=0.3) (Table 1).
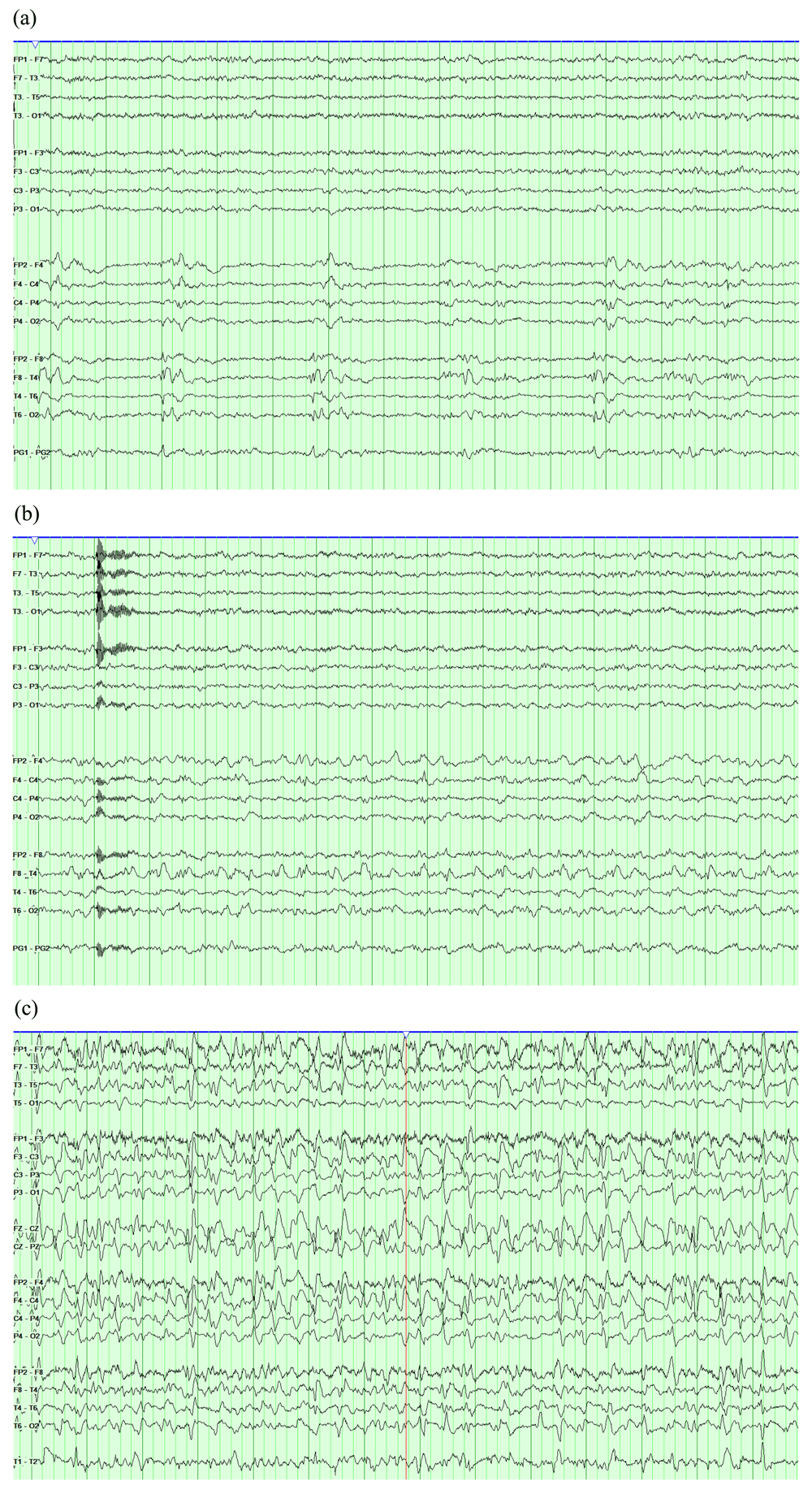
EEGs are displayed in a longitudinal bipolar montage with a sensitivity of 7 uV/mm and a time base of 30 mm/second. The high frequency filter is set to 70 Hz and the low frequency filter is set to 1 Hz. (a) Right temporal lateralized periodic discharges (LPDs). (b) The same record showing evolution of the LPDs into 2.5 Hz rhythmic theta activity, fulfilling seizure criteria. (c) Another record showing very long runs of generalized spike and wave activity that recur for most of the recording, fulfilling criteria of non-convulsive status epilepticus.
| Variable | Mortality | Hospital Stay | ||||
|---|---|---|---|---|---|---|
| Dead at 60 days | Alive at 60 days | Hospital stay <1 week | 1 week-1month | >1month | ||
| NCS | No | 40 | 110 | 44 | 60 | 44 |
| Row% | 26.7% | 73.3% | 29.3% | 41.3% | 29.3% | |
| Col.% | 64.5% | 78.6% | 78.6% | 75.6% | 68.8% | |
| Yes | 22 | 30 | 12 | 20 | 20 | |
| Row% | 42.3%* | 57.7% | 23.1% | 38.5% | 38.5% | |
| Col.% | 35.5% | 21.4% | 21.4% | 24.4% | 31.2% | |
| RPPs | No | 16 | 48 | 26 | 20 | 18 |
| Row% | 25% | 75% | 40.6% | 31.2% | 28.1% | |
| Col.% | 25.8% | 34.3% | 46.4% | 24.4% | 28.1% | |
| Yes | 46 | 92 | 30 | 62 | 46 | |
| Row% | 33.3% | 66.7% | 21.7% | 44.9% | 33.3%* | |
| Col.% | 74.2% | 65.7% | 53.6% | 75.6% | 71.9% | |
The practice of using CEEG in the ICU has developed rapidly over the past decade, particularly in North America and Europe1,5. This study is one of the first to report the experience of using ICU CEEG in Saudi Arabia, a country with a rapidly developing healthcare system that faces economic constraints. The data are consistent with prior knowledge and experience from other countries that CEEG is effective in detecting NCS and other likely harmful subclinical EEG patterns on the ictal-interictal continuum5,6. This study reveals statistically significant associations between NCS and mortality and between RPPs and longer hospital stays, supporting the clinical gestalt of identifying and treating potentially harmful CEEG patterns. However, the incurred excess risks of morbidity and mortality in patients with NCS or RPPs was relatively modest. Such modest increases are not likely to be of clinical significance to clinicians evaluating patient prognoses. Nonetheless, this study was a retrospective study with limitations that preclude definitive conclusions about morbidity and mortality risk magnitudes. These are better assessed by prospective studies and in well-developed CEEG programs.
Prior studies have not definitively proven that utilizing CEEG leads to better outcomes2,5. This, coupled with the significant resources required to effectively run an ICU CEEG program2, may lead decision makers in developing healthcare systems to hesitate to support the development of CEEG practices and research. This study presents data from a small and developing program to demonstrate real-world effectiveness of CEEG in detecting potentially harmful electrophysiological patterns. In addition, the study highlights the uncertainties regarding the clinical significance of the prognostic information provided by CEEG. Hopefully, this should lead to further development of ICU CEEG programs with embedded prospective, patient-centered research programs. Such research should focus on how CEEG may be used effectively and optimally and how the generated data may impact clinical decisions and patient outcomes in the ICU.
This study is a retrospective analysis with limitations. Retrospective EMR data did not contain accurate information with regards to the extent and evolution of mental status changes relative to the timing of the CEEG changes, use and titration of sedatives, and other management decisions. It was difficult to ascertain the timing of EEG initiation in relation to these dynamic variables of interest. Physicians did not follow a clear protocol when deciding the duration of the CEEG study. Furthermore, a selection bias is introduced because of the lack of EEG technologists at night. Longer studies may lead to higher detection rates of relevant CEEG patterns. Although the total number of cases was 202, the number of cases in most diagnostic categories was not high enough to permit subgroup analyses. The clinical setting is that of a developing program with limited resources and must be interpreted in this context. Further studies from developing healthcare systems like Saudi Arabia’s are needed to illuminate how the practice of CEEG monitoring may be best utilized to provide clinically meaningful data while caring for patients in the ICU.
Open Science Framework: The yield of continuous EEG monitoring in the ICU at a tertiary care hospital in Saudi Arabia: A retrospective study. https://doi.org/10.17605/OSF.IO/Q56J34.
This project contains all raw de-identified data associated with this study.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Neurocritical Care; Pediatric ICU
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: EEG
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: Researcher in the 'Pediatric Status Epilepticus Research Group' (PSERG); Journal reviewer; Senior Editor
Reviewer Expertise: Pediatric Neurocritical Care; Pediatric ICU
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: EEG
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 18 Oct 19 |
read | read | |
|
Version 2 (revision) 16 Sep 19 |
read | read | read |
|
Version 1 15 May 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)