Keywords
heart failure, health-related quality of life, systematic literature review, humanistic burden, economic burden, healthcare cost, hospitalization, readmission rates
heart failure, health-related quality of life, systematic literature review, humanistic burden, economic burden, healthcare cost, hospitalization, readmission rates
In response to comments from the reviewers we made the following amends to the manuscript.
Introduction
- Defined humanistic burden as used in the context of this publication
- Included introductory text on the difference between heart failure with reduced ejection fraction and heart failure with preserved ejection fraction.
- Introduced the fact that patients with heart failure experience worse health-related quality of life than those with other chronic disease
Methods
- Clarified that the systematic review that informs this manuscript was not prospectively registered with the National Institute for Health Research International Prospective Register of Systematic Reviews (PROSPERO); however, the protocol is available from the corresponding author
Discussion
- Clarified the intention of this review was to provide an overview of the humanistic and economic impact of heart failure that supplements the published literature
Defined humanistic burden as used in the context of this publication
Introduced discussion on the limited treatment options available to patients with heart failure with preserved ejection fraction
Conclusions
- Clarified that our findings suggest that both humanistic and economic burden is increased in patients with heart failure compared with patients without heart failure, rather than those with other chronic diseases as there were not sufficient data identified to support this statement
- Discussed the challenges of quantifying the detriment experienced by patients with heart failure from a broad evidence base and provided an overarching assessment of the literature
See the authors' detailed response to the review by Gerhard Wikström
See the authors' detailed response to the review by Michael McGee
Heart failure (HF) is estimated to affect more than 23 million people worldwide1 and is increasing in prevalence2. Causes of HF include those of cardiac aetiology in addition to chronic diseases such as diabetes mellitus (DM) and chronic kidney disease (CKD)3; HF has been reported as the most common cardiovascular complication of DM4. The growing prevalence of HF can be attributed both to the increasing median age of populations worldwide and to the increasing prevalence of cardiovascular disease, obesity, and DM1,4. Early treatment of DM could have a significant impact in preventing the development of HF in a proportion of patients, limiting both the humanistic burden, which we consider for the purposes of this review as the effect of HF on the health-related quality of life (HRQoL) of the individuals affected5, and the economic impact of the disease.
HF is chronic and progressive in nature, and can be classified into New York Heart Association (NYHA) classes I–IV based on physical limitation and objective assessment of the presence of cardiovascular disease6 or according to percentage ejection fraction7. The pathophysiology of HF with reduced ejection fraction (HFrEF) differs from that of HF with preserved ejection fraction (HFpEF), with a higher prevalence of non-cardiac morbidities (including DM and hypertension) in patients with HFpEF than in those with HFrEF. Given the differences in aetiology and progression of these diseases7, the economic burden of HFrEF and HRpEF may vary8–10. The signs and symptoms of HF include breathlessness, swelling, fatigue, and fluid retention, which can lead to reduced mobility and impaired daily physical functioning11. Patients may also face psychological problems, such as depression or anxiety, as well as social concerns, especially regarding isolation12. The impact of HF symptoms upon multiple aspects of patients’ lives means that the condition can cause a significant reduction in HRQoL13. Impaired HRQoL in patients with HF compared with those with chronic disease or healthy individuals is well documented14, and directly surveying the patient’s daily wellbeing is key to understanding the impact of disease on their daily life14,15. Both HF-specific and generic HRQoL instruments can be used to measure the effects of HF on patients’ daily lives and well-being16.
Most patients with HF require routine management of their disease13. Treatment typically comprises daily medication as well as periodic visits to primary care providers; however, the most economically costly aspect of the disease is admission to hospital and subsequent inpatient care17. This is often required owing to worsening of symptoms. The acute nature of these occurrences, along with the risk of infection, can mean that patients with HF may be admitted to hospital as an emergency case18, incurring high costs19. Patients are typically treated in the intensive care unit, with clinical stabilization and symptom improvement as the primary focus following admission18. Once a patient’s condition has stabilized, they are likely to be transferred to the ward, and a multidisciplinary approach employed to aid disease management18. Treatment optimization and the management of both cardiovascular and non-cardiovascular comorbidities play a significant role in preventing further hospitalizations, because hospitalization with HF is associated with high rates of readmission18. Outside hospital, many patients with HF receive care in a primary setting or may receive informal care and support in the home, imposing a wider societal cost20.
This systematic review (SR) was conducted to inform understanding of the humanistic burden and economic impact of HF by identifying relevant evidence on HRQoL, costs, and medical resource use in patients with HF.
A systematic search was performed using MEDLINE® and MEDLINE In-Process, Embase, and the Cochrane Library via Ovid between January 2002 and May 2017 for the humanistic burden SR, and between January 2007 and May 2017 for the economic impact SR. Supplementary searches included reviews of congress abstracts between 2015 and 2017 (or the most recent 2 years available) for the following meetings: International Society for Pharmacoeconomics and Outcomes Research US and European congresses, American Heart Association Scientific Sessions, European Society of Cardiology (ESC) Congress, World Congress of Cardiology and Cardiovascular Health, American College of Cardiology Annual Scientific Session, and ESC Heart Failure. The search strings used to identify evidence are listed in Table 1. This review was not prospectively registered with the National Institute for Health Research International Prospective Register of Systematic Reviews (PROSPERO), but the protocol is available on request from the corresponding author.
Abstracts and titles identified were screened by an independent reviewer to determine whether they met the PICOS (patient, interventions, comparisons, outcomes, and study design) eligibility criteria (Table 2), in accordance with 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines21. All publications that met the entry criteria for the review were obtained as full articles and reassessed against the review criteria. Owing to the large number of citations meeting the predefined inclusion criteria, a decision was made to restrict data extraction from eligible studies. For both SRs, data were extracted only from full publications of observational studies. Data were not extracted if the study population included fewer than 200 patients for the humanistic burden SR, and fewer than 100 patients for the economic impact SR. The restriction criteria used are summarized Table 2.
HF, heart failure. HRQoL, health-related quality of life. NYHA, New York Heart Association. SR, systematic review.
In the initial searches, 11 622 papers were identified, of which 2186 were removed as duplicates, and 9436 papers were included for electronic screening. Electronic screening identified 8166 papers that did not meet the inclusion criteria. In total, 124 papers were identified for inclusion following full paper review: 54 papers reporting HRQoL and 71 papers reporting costs and resource use (including one reference that reported data for both outcomes). A PRISMA flow diagram is shown in Figure 1. The studies identified were grouped according to the key data presented, as shown in Figure 2.
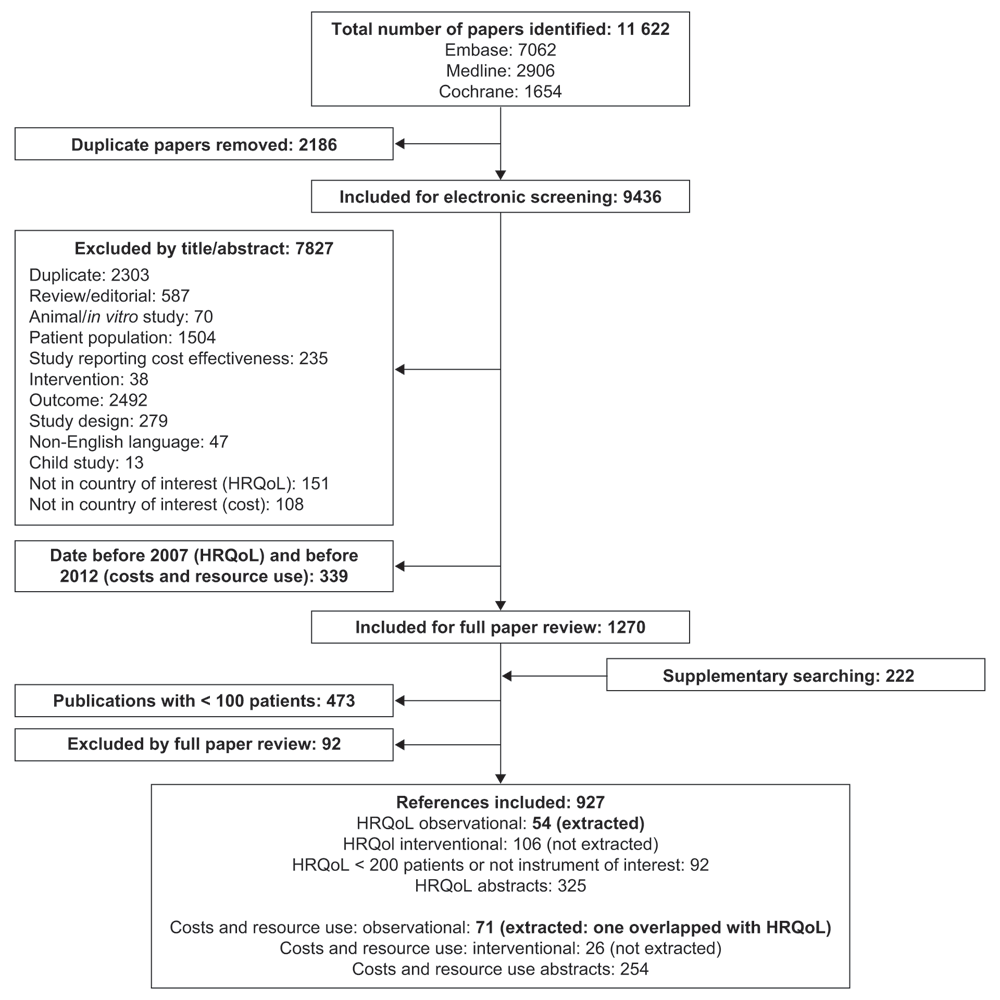
HRQoL, health-related quality of life. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
A small number of studies assessed HRQoL in patients with HF compared with individuals without HF, or patients with other chronic diseases. In a study conducted in the Netherlands, patients with HF (NYHA class II–IV) reported worse scores across all domains of the 36-item Short-Form Health Survey (SF-36) than a population of age- and sex-matched community control individuals (Figure 3)22. A Danish study also found that patients who had been discharged from hospital reported lower 12-item Short-Form Health Survey (SF-12) physical component summary (PCS; mean±standard deviation [SD]: 42.5±11.1 vs 47.7±13.4, p<0.001) and mental component summary (MCS; mean±SD: 48.2±10.9 vs 51.5±14.7, p<0.001) scores than a reference group representative of the national population23. A further cohort survey, conducted in the UK, compared patients with HF with those who had asthma, chronic obstructive pulmonary disease (COPD), DM, epilepsy, or stroke. Patients with HF reported lower baseline 5-dimension EuroQol questionnaire (EQ-5D) utility and visual analogue scale (VAS) scores than patients with asthma, DM, epilepsy, and stroke, and similar scores to patients with COPD24.
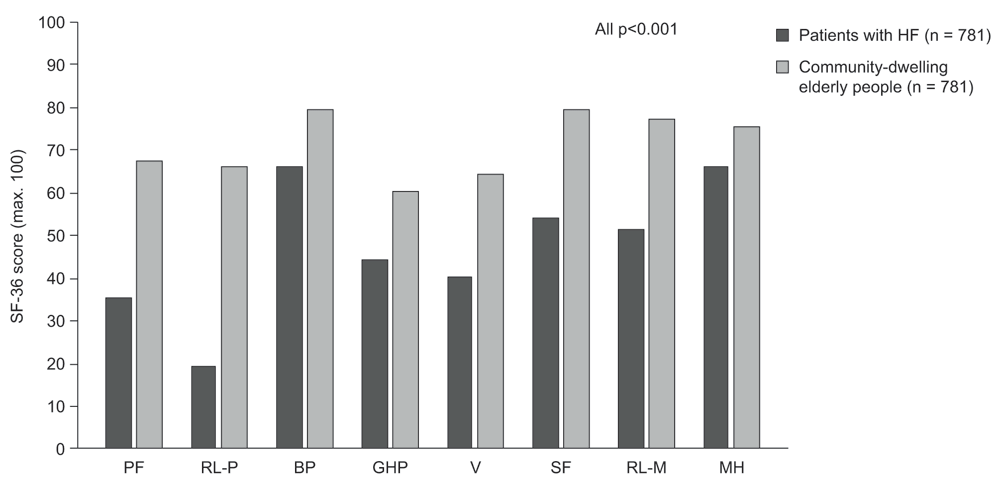
This figure provides a graphical representation of the component scores for the SF-36 HRQoL questionnaire for patients with HF (stage II–IV) compared with community-dwelling elderly people who do not have HF. BP, bodily pain. GHP, general health perception. HF, heart failure. HRQoL, health-related quality of life. MH, mental health. PF, physical functioning. RL-M, role-limiting (mental). RL-P, role-limiting (physical). SF, social functioning. SF-36, 36-item Short-Form Health Survey. V, vitality.
Studies from Sweden25, Australia26, and the USA27,28 assessed HRQoL by NYHA HF class and reported a greater humanistic burden with more severe disease (Figure 4). Based on the evidence identified, there was a reduction in HRQoL between NYHA HF classes I and II25,27,28 and classes II and III25–28, and between classes III and IV25–28, assessed using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the Kansas City Cardiomyopathy Questionnaire (KCCQ), both of which are cardiac-specific instruments. One study also reported decreasing SF-36 PCS and MCS scores with increasing NYHA HF class25. For SF-12 scores, there was a greater reduction between NYHA classes I and II, and smaller reductions between more severe NYHA classes (Figure 4). A consistent decrease in utilities between classes II and III (–0.042) and classes III and IV (–0.041), measured using the 5-level EQ-5D (EQ-5D-5L) was also reported by a single study alongside a decline in MLHFQ scores (Figure 4)26.
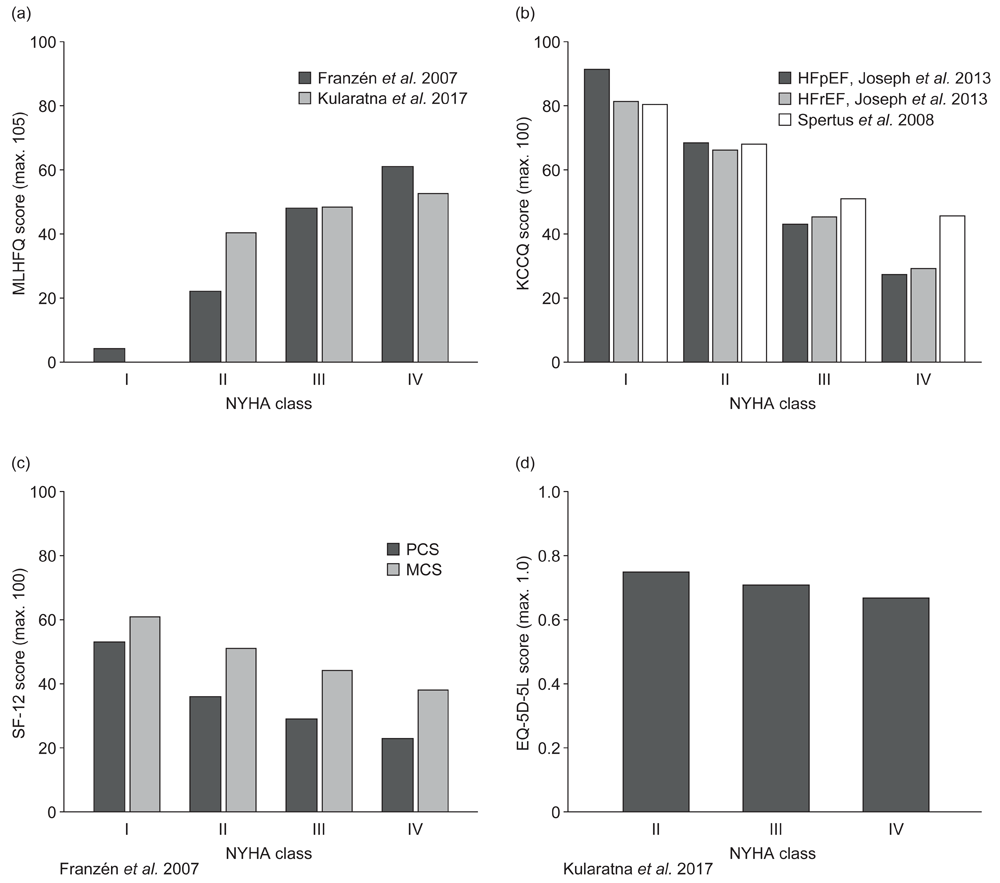
Humanistic burden with increasing New York Heart Association class measured using: (a) MLHFQ25,27, (b) KCCQ26,28, (c) SF-3625, and (d) EQ-5D-5L26.
This figure provides a graphical representation of HRQoL scores by NYHA class as measured by the MLHFQ, KCCQ, SF-36, or EQ-5D-5L questionnaires. Better health-related quality of life is represented by higher KCCQ, SF-36, and EQ-5D-5L scores, and lower MLHFQ scores. EQ-5D-5L, 5-level, 5-dimension EuroQol questionnaire. HFpEF, heart failure with preserved ejection fraction. HFrEF, heart failure with reduced ejection fraction. HRQoL, health-related quality of life. KCCQ, Kansas City Cardiomyopathy Questionnaire. MCS, mental component summary. MLHFQ, Minnesota Living with Heart Failure Questionnaire. NYHA, New York Heart Association. PCS, physical component summary. SF-36, 36-item Short-Form Health Survey.
Two further studies, both conducted in the USA, reported a negative correlation between HRQoL and increasing HF severity. A study of patients with HF living in a rural location reported a negative association between NYHA HF class and MLHFQ score, but did not report results by separate NYHA classes29. Another study investigated HRQoL in a subgroup of patients with HF as a comorbidity, from a population with CKD in a subanalysis of the Hemodialysis Study, a randomized controlled trial of patients undergoing haemodialysis. Reductions in both mental and physical HRQoL were reported with increasing HF severity (categories ranging from ‘absent’ to ‘severe’). Scores in the role-limiting (physical), bodily pain, general health, vitality, social functioning, and mental health domains, as well as overall PCS scores, decreased with increasing HF severity30.
Few longitudinal studies reporting HRQoL in HF were identified. Of the studies that were purely observational, two reported a decrease in HRQoL over time, whereas the remaining three reported an improvement in HRQoL across the study period. A UK cohort study investigating HRQoL in patients with HF relative to those with other chronic diseases reported generic and disease-specific HRQoL scores at baseline and 1 year. Patients with HF were the only group to report a reduction in HRQoL over the follow-up period, with significantly lower EQ-5D VAS scores indicating worse HRQoL at 1 year24. A US study reported HRQoL over a follow-up of up to 6.6 years, adjusting for the effect of time on disease deterioration30. PCS scores decreased over time, along with scores for six of the eight SF-36 domains; MCS scores and scores for the role-limiting (mental) domain did not decrease over time30.
A study in the Netherlands, however, reported that patients with a higher level of education experienced a statistically significant improvement in the role-limiting (emotional) domain of the SF-36 across an 18-month longitudinal study (p=0.015). No significant difference was found in the remaining SF-36 domains31. Patients in a Canadian study in whom HF was diagnosed following an emergency department (ED) visit reported increases in physical HRQoL scores over a 12-month period (SF-12 PCS score: 1.6–3.4; KCCQ-physical limitation score: 5.6–9.4)32. An analysis modelled health trajectories over 12 months defined by patients’ KCCQ scores over time using data from the Patient-Centered Disease Management for Heart Failure trial. Patients with a poor or moderate health status trajectory experienced improvements in KCCQ scores between baseline and 3 months followed by a stabilization in scores up to 12 months, whereas those who had a trajectory of ‘marked improvement’ reported an increase in HRQoL over the whole 12-month period33.
In a number of the longitudinal studies identified, healthcare professionals provided support to patients over the follow-up period, which was considered likely to impact HRQoL. These studies are summarized in Extended data: Supplemental Table 134. Studies in Norway35 and in Spain36 reported a statistically significant improvement in MLHFQ scores between baseline and 6 or 12 months, respectively, for patients monitored over the study period. In the Spanish study, patients continued to experience an increase in HRQoL over 5 years36. An improvement in MLHFQ scores over 12 months following enrolment into an HF clinic was also reported by patients in a Canadian study37.
Few studies comparing HRQoL in patients with HFpEF and those with HFrEF were identified. In a study conducted in Germany, patients with HFpEF reported better physical functioning, measured using the SF-36, than patients with HFrEF38. A study conducted in the USA reported that patients with HFpEF experienced no greater impact on HRQoL than those with HFrEF as disease severity increased. However, patients with HFpEF, with KCCQ scores in the lowest quartile, had a higher risk of mortality or hospitalization as well as lower rates of event-free survival than those who had scores in the highest quartile27.
Comorbidities in patients with HF were typically associated with reductions in HRQoL (Extended data: Supplemental Table 134). In studies conducted in Spain and the USA, respectively, patients with HF and iron deficiency39 or pain40 reported higher overall and physical summary MLHFQ scores, representative of worse HRQoL, than those without these comorbidities. However, a further study in the USA found that although patients with anaemia reported worse baseline KCCQ scores than those without, deterioration in HRQoL over a 3-month period was similar in both populations28. Patients in a study in the Netherlands who experienced difficulties with sexual activity reported worse HRQoL than those without difficulties41. In a US study, excessive daytime sleepiness and cognitive impairment were associated with comparatively lower HRQoL42. In a second US study, patients who had comparatively lower haemoglobin levels at baseline reported worse KCCQ and MLHFQ scores than those with higher haemoglobin levels43.
Patients in Australia, the UK, Ireland, the USA, and Norway who had ischaemic heart disease with ischaemic HF, angina, or a myocardial infarction as a comorbidity reported PCS scores that were lower than expected for the general population (more than one SD below the standardized mean of the general US population), whereas MCS scores were within one SD of the mean44.
Poor mental health was generally associated with worse HRQoL in patients with HF. Comparative studies were identified that focused on depression, suicidal ideation, panic disorder, and distress (Extended data: Supplemental Table 134).
Patients with HF and depression had worse HRQoL than those without depression in studies from Germany, Italy, and the USA. Statistically significant reductions in scores across all SF-36 domains and the KCCQ summary domains were found with increasing severity of depression in two studies in Germany45,46. A further German study reported an association between lower SF-36 physical functioning (PF) and bodily pain scores as severity of depression increased47. Furthermore, in a study in the USA, PF was negatively correlated with depression48. Worse health status, as defined by KCCQ score, was associated with a comparatively higher risk of depression at baseline in another US study33; and a German study indicated that patients with type D personality, predictive of depression, reported worse HRQoL (MLHFQ scores) than those with a different personality type49.
Suicidal ideation and ideas of self-harm were reported to be associated with reduced HRQoL50. Patients with panic disorder51 and those who experienced distress at baseline and/or follow-up52 reported worse HRQoL than those who did not. Another study reported an association between patients’ end-of-life preferences and their physical HRQoL (SF-36 PCS scores)53.
Patients’ perceived health status was reported to have a moderate impact on MLHFQ score in a Canadian study54, and social functioning, measured using the KCCQ, was found to be a predictor of perceived health status, particularly in male patients with HF in the USA55. High levels of self-care were associated with better MLHFQ or KCCQ scores in studies in Italy56 and the USA57, respectively, as well as lower hospitalization rates in the Italian study.
Patients hospitalized for HF experienced comparatively better HRQoL once discharged from hospital (Extended data: Supplemental Table 134). A study in the USA and Canada found that EQ-5D scores were higher at the time of hospital discharge than when patients were admitted to hospital (baseline), and remained higher than baseline 30 days after discharge from hospital58. Another US study showed that MLHFQ scores improved significantly during the first month post-discharge (p<0.001)59. Furthermore, patients in a study in the USA who did not experience readmission to hospital within 30 days of discharge experienced better HRQoL (KCCQ scores) than those who did60. Fear of hospitalization had a negative impact on patients’ MLHFQ scores in an Italian study61, and unplanned hospitalization was identified as a predictor of better SF-12 PCS scores in a patient population who had previously been hospitalized for HF in a study in Denmark23.
Owing to the chronic nature of HF, the implementation of appropriate disease management is an important part of treatment, and evidence identified in this SR indicates that it is associated with HRQoL improvements (Extended data: Supplemental Table 134). Patients in the UK62 and Germany63 with HF and either atrial fibrillation or sinus rhythm reported an improvement in HRQoL following cardiac resynchronization therapy and treatment with β-blockers, respectively. The use of left ventricular assist devices (LVADs) as bridging or destination therapy was also associated with an improvement in HRQoL in a US study64, and patients with an LVAD had better HRQoL than patients assessed for, or awaiting, heart transplant in a UK study65.
Several studies were identified that reported HRQoL as a predictive factor both of mortality and of outcomes in patients with HF (Extended data: Supplemental Table 134). Scores in the SF-36 and SF-12 general health and PF domains were predictors of mortality in studies conducted in the Netherlands66 and the USA67, and were associated with an increased risk of hospitalization and ED visits in another study in the USA68. Two further studies, one in Italy69 and the other multinational70, found that KCCQ score was a predictor of mortality, and a study in the USA reported a negative association between MLHFQ score and cardiac event-free survival71. Physical and depressive symptoms, in addition to spiritual well-being and comorbidity count, were negatively correlated with HF-specific health status (partially determined by KCCQ score) in patients in the USA72. An Australian study examined the contribution of MLHFQ and EQ-5D scores to composite scoring systems in HF trials73.
In several studies, patient ethnicity and sex were shown to impact HRQoL (Extended data: Supplemental Table 134). A study in the USA found that Hispanic patients reported better HRQoL than those of white or black ethnicity74. In two Canadian studies, women reported worse physical and overall HRQoL, respectively, than men at baseline32,75, and worse physical HRQoL at 12 months75. Sociodemographic factors including marital status, education, income, and employment were correlated with worse HRQoL in studies in the Netherlands31 and in Canada, France, and the USA76.
The overall costs associated with the treatment for HF varied widely across studies. This is likely to result from disparities in study settings and populations, which meant that the patients included differed in terms of disease severity, clinical history, and comorbidities.
Five studies conducted in Europe reported total annual healthcare costs associated with HF. The lowest annual per-patient costs reported were €3150 in a German study77, followed by €5700 in a Swedish study78, and €6571 in a study conducted in Spain79. Two Italian studies, each of which included only patients who had experienced an HF-related hospitalization, reported total annual costs of €11 10080 and €11 86481, respectively.
The lowest total annual costs reported in the USA were for a subpopulation of individuals enrolled in Medicare; patients with HF who were not classed as having a low income or dual eligibility for Medicare and Medicaid (non-low-income/dually eligible [LI/DE] cohort) incurred per-patient costs of US$13 897 annually. This value was US$17 840 for patients with HF who were classed as LI/DE82. The highest annual US costs identified were from a study comparing patients with HF who died within 1 year and those who survived. Surviving patients incurred total mean per-patient costs of US$36 426 per annum83. A further four US studies reported annual per-patient costs in the range of US$16 912–29 45684–87. Two additional US studies recorded total costs for patients in the period before they died as a result of HF; these data are not included in the overall range, but are presented in Extended data: Supplemental Table 288–90. In addition to evidence from Europe and the USA, total annual costs associated with HF were reported as C$27 809 in a Canadian study91 and ¥28 974 in a study from China92.
Rather than reporting annual total costs for HF, two studies presented data collected over different time periods (Extended data: Supplemental Table 290). A UK study reported the total costs incurred in the year before an HF event and in the 36 months after the event93, and a US study reported total Medicare payments over 30 days for patients discharged from hospital, grouped based on dyspnoea severity94.
Eight studies, all from the USA, compared costs and resource use for patients with HF with those for individuals without HF, or with other chronic diseases. Total annual costs were significantly higher for patients with HF than for those without HF (p<0.001): 4.6 times higher (US$27 152 vs US$5952) during 2010–2011, and 4.3 times higher during 2002–2011 (US$23 854 vs US$5511)85. A breakdown of constituent costs in this study is shown in Figure 5. Another study compared allowed monthly Medicare costs and resource use for patients with HF and all individuals who made Medicare claims (fee-for-service population). Per-patient allowed monthly costs for HF were 3.2 times higher than those incurred by the average fee-for-service patient (US$3395 vs US$1045)95. Patients with HF also had comparatively higher rates of inpatient admission, 30-day readmission, and use of skilled nursing facilities, meaning that higher costs were incurred for all of these resources, compared with the Medicare fee-for-service population.
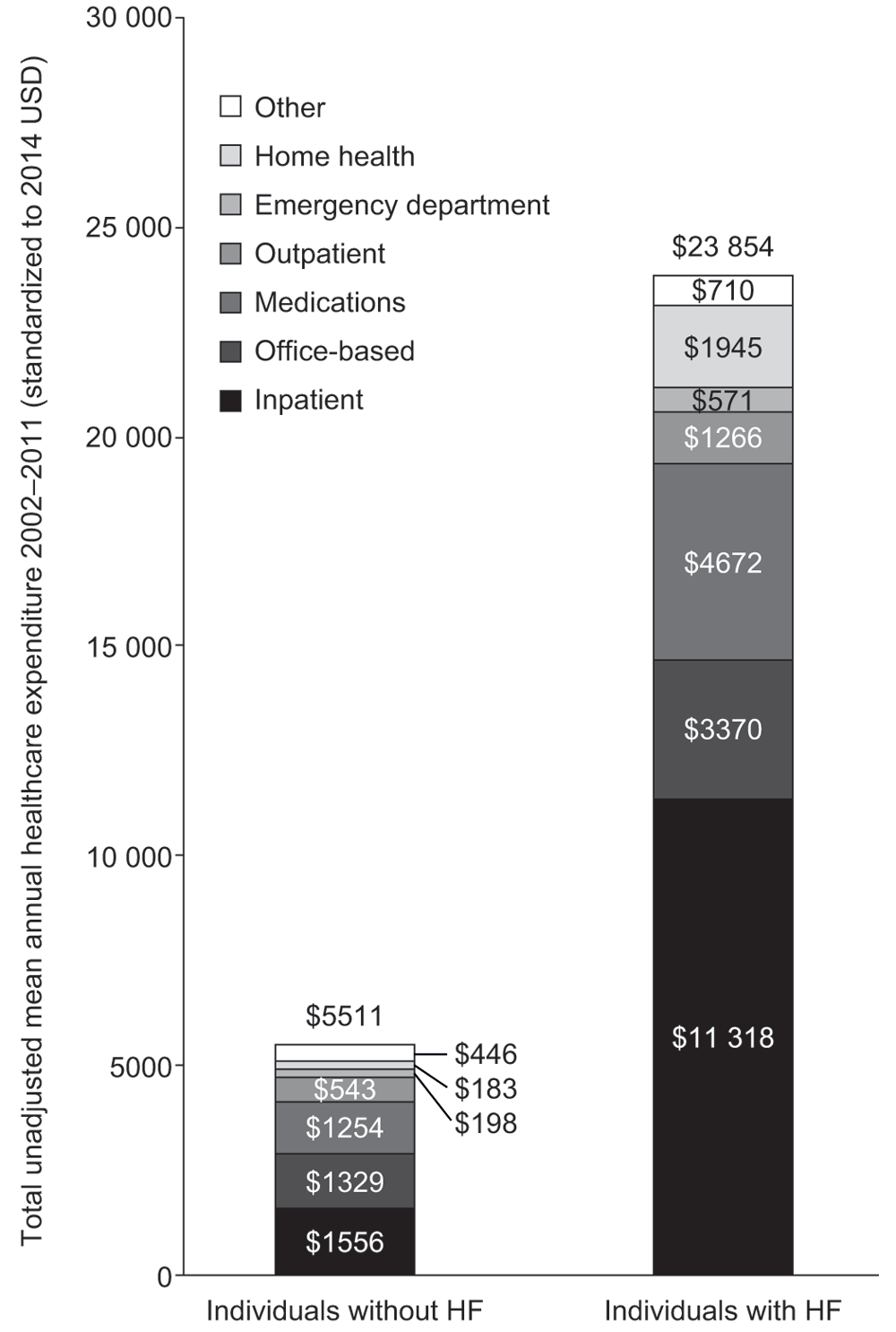
This figure provides a graphical representation of annual healthcare expenditure with constituent costs for individuals with or without HF between 2002 and 2011. HF, heart failure.
Additional evidence indicated that HF is associated with increased resource use. A study of patients who were admitted to hospital for trauma and subsequently discharged found that the presence of HF was associated with a 71% increased risk of 30-day hospital readmission (24% vs 14%; p<0.0001)96. An examination of the resource use associated with hospital discharge for patients with HF, compared with all patients discharged, showed that costs for cardiology, supplies and devices, coronary care, and operating room services were 1.3–3.3 times higher for patients with HF97. Although few studies focused on indirect costs, one study examining care requirements was identified. In patients aged over 50 years, individuals with HF were significantly more likely than those without HF to make use of either formal (paid) care (HF: 9.1%; no HF: 1.5%; p<0.001) or informal care (HF: 33%; no HF: 8.6%; p<0.001), defined as care provided by a family member or unpaid volunteer98. Patients with HF required significantly more informal care per week than those without HF (32.1 hours vs 25.1 hours; p=0.002).
Three studies compared the costs associated with HF and those for other chronic diseases. One study showed that similar total annual costs were associated with HF and obesity (US$1642 and US$1908, respectively); however, these conditions were considerably more costly than hypertension, which was associated with costs of US$431 per annum99. A second study reported that total annual costs were nearly twofold higher for HF than for DM, partly owing to higher inpatient and outpatient costs87. Finally, a study reporting disease-specific costs showed that HF incurred higher annual medical and pharmacy costs than asthma, coronary artery disease, COPD, DM, hyperlipidaemia, and hypertension82.
Three studies showed that HF is associated with additional costs in patients with DM. A UK study estimated that annual inpatient care costs for a typical 60-year-old man with type 2 DM are more than six times higher in the presence of HF than if HF does not occur (£3191 for HF vs £459 for no HF)100. The occurrence of HF in a patient with DM was also estimated to result in 84% higher non-inpatient costs. The cost of preventable hospitalization for HF in patients with DM was reported as US$7949 in a US study101, and a second US study found that acute care for an HF episode incurred costs of US$23 758 per event-year, plus US$1904 in ongoing management costs102. The impact of HF in obese patients was also examined in one study. Patients with obesity but no HF incurred annual total costs of US$1908; however, this rose to US$5276 in patients with HF as well as obesity99.
Two US studies reported the cost impact of comorbidities in patients with HF (Extended data: Supplemental Table 290). Shaya et al. estimated the annual cost savings that could be made via a 20% reduction in a range of comorbidities, the most costly of which were cardiovascular disease and DM103. Smith et al. estimated the contribution of various comorbidities to the overall cost of care for patients with HF, finding that DM incurred the greatest costs of the comorbidities examined in the study104.
Only one study, conducted in Japan, reported costs by HF severity105. Hospitalization costs were modelled across NYHA classes: compared with NYHA class II, classes III and IV were associated with an additional US$490 and US$640, respectively (p<0.001).
Two Swedish studies collected data on the total annual direct costs associated with HFrEF106 and HFpEF107. These studies, however, were not conducted simultaneously or designed to compare costs by ejection fraction, so limited inference should be drawn from across-study comparisons. HFrEF was associated with slightly higher annual costs than HFpEF (€12 447 vs €11 344), as a result of higher costs associated with inpatient care, hospitalizations, and medication. Annual outpatient clinic costs were approximately 43% higher for HFpEF than for HFrEF (€1561 vs €1094).
Two US studies compared resource use between patients with HFrEF and those with HFpEF. One study found no significant difference between the two groups in terms of 30-day hospital readmission rate. However, patients with HFrEF had a significantly greater length of stay (LoS) in hospital than patients with HFpEF (HFrEF: 10.9 days; HFpEF: 8.5 days; p=0.027)96. In a second study, there were no significant differences between groups in terms of adjusted 30-day readmission rates, annual hospitalization rate, LoS, or pharmaceutical dispenses. Although the absolute differences between cohorts were small, HFpEF was associated with significantly more outpatient visits (HFpEF: 21.5; HFrEF: 20.1; p<0.002) and ED visits (HFpEF: 3.24; HFrEF: 2.94; p<0.002) annually than HFrEF, as well as a significantly higher rate of readmission within 1 year (HFpEF: 58%; HFrEF: 55%; p=0.010)108.
Inpatient care and hospitalization were identified as major cost drivers in HF and were reported as the single largest contributor to costs in multiple studies across different geographies. There was relatively wide variation across studies in the percentage of costs contributed by inpatient care, which can be accounted for by differences between populations and disparities in the types of costs included in each study.
In total, five European studies presented inpatient care or hospitalization as a percentage of total or direct costs: one each from Germany77 and Italy81 and three from Sweden78,106,107. Inpatient care or hospitalization contributed 69–87% of total costs in these studies (Extended data: Supplemental Table 390). Ten studies reported relevant data from North America, of which eight were conducted in the USA83,85–88,95,109,110, one took place in Canada91, and one conducted analyses using data from a clinical trial with study centres in the USA, Canada, and France111. The percentage of total costs contributed by inpatient or hospitalization costs across these studies varied between 47%85 and approximately 87%106,109 (Extended data: Supplemental Table 390). Three studies included only patients who died from HF; however, the contribution of inpatient care to total costs in these studies fell within the overall range for all studies83,88,111. An additional US study reported the proportion of total claims for HF accounted for by inpatient claims (60.5%) and outpatient claims (39.5%)99. One additional study, conducted in China, reported that inpatient care contributed 66% of total costs (Extended data: Supplemental Table 390)92.
A German study estimated the future costs of inpatient care for HF, predicting that the overall budget impact in Germany would be €1.80 billion in 2025, up from €1.27 billion in 2009112. This predicted increase arose from a 14% increase in the cost allowance per patient with HF in hospital and a 23% increase in the number of patients with HF in hospital.
Several other studies reported inpatient or hospitalization costs (Extended data: Supplemental Table 290). Seven studies, two from the UK100,113 and five from the USA19,114–117, collected data on overall inpatient/hospitalization costs associated with HF; however, these were not reported as a percentage of total costs. Additionally, five US studies reported the costs associated with a single hospitalization118–122, and one reported the cost of an ‘acute HF hospital episode’ as US$10 775123.
Patients’ risk of hospitalization for HF or readmission within 1 year is likely to be influenced by factors such as disease severity, and consequently a wide range of rates were reported across the studies identified. There was a larger evidence base for all-cause 30-day readmission rates; these studies indicated that between 15% and 30% of patients hospitalized for HF are likely to be readmitted within a month after discharge from hospital.
Seven studies79,80,82,87,109,111,124 reported 1-year hospitalization rates (Extended data: Supplemental Table 490). An Italian study found that HF-related and all-cause hospitalization rates were considerably lower in patients who had never been hospitalized for HF than in those who had (HF-related: 0.4% vs 22%; all-cause: 15% vs 59%)80. All-cause hospitalization was 30.8% in a Spanish study79. In the USA, HF-related hospitalization rates ranged from 6%82 to 22%109, and all-cause hospitalization rates ranged from 33% to 57%109. In a multinational study, 38% of patients experienced all-cause hospitalization111. A study in China reported relatively high rates of HF-related hospitalization within 1 year, at 34.8%124. A further 13 studies reported data on hospitalizations (Extended data: Supplemental Table 290)78,95,106,107,113,115,116,121,122,125–128, but did not present data as a percentage of patients experiencing hospitalization, did not report rates over 1 year, or included only patients who died of HF.
Studies that reported 1-year hospital readmission rates, of which five were from the USA86,108,118,129,130, and one each from Italy81, Australia131, and China92, are summarized in Extended data: Supplemental Table 590. HF-related readmission rates ranged from 13.8% in a US study130 to 46.1% in the Italian study81, and all-cause readmission rates ranged from 55% in the USA108 to 73% in Australia131.
Hospital readmission rates at 30 days were reported in studies from the USA, Australia, and China (Extended data: Supplemental Table 690). In the USA, HF-related rates of readmission were 6.4–12.5%94,120,121,129,132,133, and all-cause rates were 17.1–30.4%86,94–96,108,114,119–121,129,130–138. An additional US study reported all-cause 30-day readmission rates after hospital discharge following implantation of an LVAD as 27.6%139. Similar rates were reported in Australia (HF-related: 11%; all-cause: 27%)131 and slightly lower rates reported in China (HF-related: 4.1%; all-cause: 16.2%)92. In addition, a study from Spain reported readmission rates in the 90 or 180 days after discharge from hospital (Extended data: Supplemental Table 290)140.
Two US studies focused on preventable readmissions. Gunadi et al. reported results from a care programme intended to reduce readmission rates, showing that the decrease in variable cost for each avoided hospital readmission was US$5652141. Chen et al. examined the rate of preventable hospital readmissions based on patients’ location and found that those in remote rural areas had a 27% lower risk of 30-day preventable readmission than patients living in urban areas142.
A small number of the studies identified reported data that did not fall into any of our key areas of interest (Extended data: Supplemental Table 290). A Spanish study reported prescription drug costs143, a study from the Netherlands reported care home and nursing home costs for patients at the end of life144, and three North American studies reported hospital LoS58, nursing contacts145, and the association between heart rate and medical costs in patients with HF146, respectively.
Given the breadth of the evidence identified and the differences in methodology, health technologies and countries in which studies were conducted, we provide an overview of the humanistic and economic impact of HF, intended to supplement the published literature.
The evidence identified in this SR illustrates that HF is associated with a substantial humanistic burden. For the purposes of this review, we consider humanistic burden to represent the impact of HF on the individual affected, as indicated by patient-reported outcomes. Patients with HF experience worse HRQoL than both individuals without HF and patients with other chronic diseases, and the burden of HF is greater for patients with more severe disease than for those at earlier stages. Poor mental health, comorbidities, and hospitalization are likely to be associated with worse HRQoL in patients with HF, whereas appropriate disease intervention can help to bring about improvements in HRQoL. This is supported by evidence that HRQoL decreases over time in the absence of disease management, but for patients who were provided support, HRQoL increased over time. Taken together with evidence indicating the positive impact of disease management and self-care, and structured support, this indicates possible ways of improving HRQoL for patients with HF.
The majority of the economic studies identified in this SR were conducted in the USA, with a smaller evidence base in Europe and relatively few studies from the rest of the world. HF is associated with considerable healthcare costs, and patients with HF incur higher costs and greater resource use, including inpatient, outpatient, and informal care, than individuals without HF or with other chronic diseases. The occurrence of HF as a comorbidity is also associated with extra costs. There is evidence to indicate that costs rise with increasing severity of HF, although this is based on only one study. The results of studies reporting costs and resource use by ejection fraction were mixed and did not provide sufficient evidence to conclude whether HFrEF or HFpEF incurs higher costs. As the diagnosis of HF has previously hinged on EF, with therapy development focused on reduced EF, limited evidence may be available on the treatment of HFpEF 147. Patients with HF are at a relatively high risk of hospitalization or readmission; accordingly, inpatient care and hospitalizations were identified as key cost drivers.
Several studies suggested that improved disease management, with the aim of reducing the number of inpatient cases and reducing patients’ risk of hospital readmission, could be a way to limit the economic impact of HF. Specific strategies discussed included use of case-management programmes, identification of risk factors for readmission, and optimization of medication, for example by improving adherence.
This SR identified a broad range of studies; consequently, this review has focused on key areas of interest that best illustrate the impact of HF. Some studies have been presented in less detail, either because they did not discuss these key themes or because the data reported were not readily comparable with the rest of the evidence base, owing to differences in study population, setting, or time frame.
Although a large number of studies were identified, some gaps in the evidence base were apparent. In studies comparing patients with HF and individuals with other chronic conditions, disease severity in the individual patient populations was not specified, nor was the impact of treatment interventions over the period of the study. This is particularly relevant when comparing different diseases, for which the effect of treatment upon HRQoL may vary considerably. Further studies on HRQoL in patients with HF, particularly those assessing the incremental effects of the disease over time or with increasing severity, would be valuable as part of an overall approach to identify means of improving patients’ well-being.
A relatively small number of studies that assessed the economic impact of HF included detailed clinical information. The majority of studies in this SR, particularly those from the USA, which was the country with the largest evidence base, identified patients with HF in administrative claims databases using International Classification of Diseases diagnosis codes. This meant that factors such as comorbidities, HF severity by NYHA classification, and disease history were not known for all patient populations. These factors can have a large impact on disease outcomes and associated costs; therefore, some of the variation between the costs reported in different studies is likely to be attributable to factors that are not recorded. Economic studies in which patients’ medical records are available might therefore be valuable to obtain more granular data on cost drivers in HF. In addition, there is little published information on the indirect costs associated with HF, with very few studies reporting data on informal care for patients with HF; thus, the wider societal impact of the disease is not immediately apparent in the literature.
The design and scope of this SR meant that it is possible that not all relevant evidence was identified. Restrictions were applied to the evidence identified in the initial searches, by patient numbers, HRQoL instrument, and type of study. In particular, the restriction to economic studies including at least 100 patients and humanistic studies including at least 200 patients may have skewed the SR toward studies using patient registries and administrative claims data. Therefore, any studies with smaller sample sizes that included detailed clinical data for patients would not have been examined within the scope of the review. A formal assessment of the studies included was not carried out as part of the review process. This would typically include evaluation of each study’s inclusion criteria, measurement methods, analytic methods, and risk of bias148. Although all evidence discussed here is of value to address our research question, such an assessment might have highlighted any studies that were of particularly high or low quality, and helped to explain any major differences between the trends reported in different studies.
Our findings indicate that both the humanistic and economic burden is increased in patients with HF compared with individuals without HF. Quantification of the detriment experienced by patients with HF is challenging owing to the heterogeneity of the study population and methodology employed as well as inherent differences between health systems and costs in different countries. However, there is overarching evidence to suggest that the burden of disease increases as disease worsens. Inpatient care and hospitalization costs were identified as key economic drivers
It appears that slowing or preventing HF progression is likely to improve patients’ overall well-being, a healthcare aim that is particularly important given the substantial and increasing humanistic burden experienced by patients with HF. Reduction in patients’ hospitalization rates, and limiting the overall requirement for inpatient care, is the healthcare goal that would have the greatest impact on the economic burden of HF. The evidence that we have identified suggests that early treatment of HF to prevent or to delay disease progression, as well as careful disease management to avoid or lessen the need for hospital admission, is likely to lessen the humanistic burden and economic impact of HF.
All data underlying the results are available as part of the article (included under extended data), and no additional source data are required to support our results.
figshare: Supplemental content 1 – Supplemental Table 1, http://doi.org/10.6084/m9.figshare.809991534.
figshare: Supplemental content 2 – Supplemental Tables 2–6, http://doi.org/10.6084/m9.figshare.809996990.
The systematic review protocol is available on request by contacting the corresponding author.
PRISMA checklist: figshare: Supplemental Content 3 – PRISMA checklist, http://doi.org/10.6084/m9.figshare.8100020149.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a collaborator in two referred papers in the reference list
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiology, perioperative medicine, indigneous health, cardiac implantable electronic devices
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 19 Aug 20 |
read | |
|
Version 1 13 Jun 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)