Keywords
Isolated Non compaction of the Ventricular Myocardium, Cardiomyopathy, Histology, Sudden Cardiac Death, Autopsy.
Isolated Non compaction of the Ventricular Myocardium, Cardiomyopathy, Histology, Sudden Cardiac Death, Autopsy.
1. Several data about the patient such as: the patient’s race (this is important due to the influence of genetic factors); how long the symptoms have occurred; symptoms other than dyspnea; the patient’s occupation; medications that have been taken; physical examination; laboratory findings; and how the patient was diagnosed with heart failure, ECG data, therapy administered were all included in the new version.
2. Histopathological findings are added.
3. Discussion was enriched with other references.
4. The role of autopsy and collaboration between cardiologists and forensics is added in the summary
See the authors' detailed response to the review by Citrawati Dyah Kencono Wungu
See the authors' detailed response to the review by Mokhles lajmi
Ventricular non-compaction (VNC) is a complex and heterogeneous cardiomyopathy first described in 1926. It is a rare disease with a reported prevalence of 0.014–0.17%1. Several terms are used to describe this disease namely: “cardiac hyper and excessive trabecularization”, “spongy myocardium”, “honeycomb myocardium”, or “persisting myocardial sinusoids”, and even “isolated ventricular abnormal trabeculation”. It is characterized by hypertrabeculation with an excessive lace-like network of trabeculae and deep trabecular pockets in the ventricle, creating a perfect environment for thrombi formation. VNC can be detected in all age groups, ranging from the fetal period to adulthood2. It can occur sporadically or is hereditary secondary to chromosomal abnormalities. It can also be associated with other cardiac diseases, which may be congenital. Additionally, VNC is represented by a large spectrum of symptoms and clinical features ranging from normal variants to pathological phenotypes. Indeed, VNC may remain asymptomatic until a complication occurs. Cardiologists must pay more attention to various clinical manifestations, including heart failure, arrhythmias and cardio embolic events, which can be related to VNC, to initiate an early treatment and avoid potentially fatal complications3.
Herein, we report a fatal case of VNC in a 20-year-old male, attested by the autopsy, and we discuss different mechanisms involved in the occurrence of death.
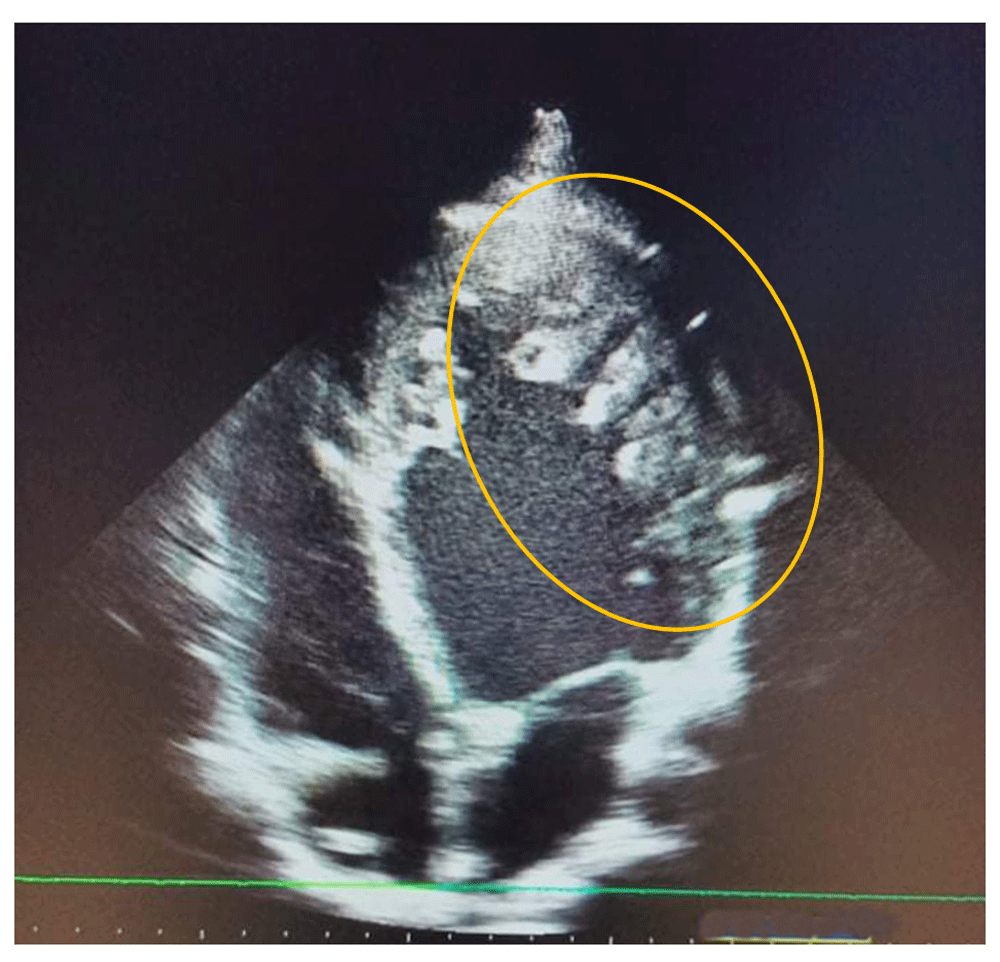
A 20- year -old Caucasian student male, who presented with dyspnea and chest pain in March 2019, was followed up by the cardiology department and classified as stage 2 on the New York Heart Association (NYHA) Functional Classification. The patient had no other relevant personal or family past medical history. The patient had 172 cm tall and weighs 62 Kg; BMI are 21 Kg/m2. Blood pressure was 120/70 mmHg with a pulse of 80 bpm. Physical examination showed no other abnormalities. ECG showed no significant abnormalities as well as laboratory findings. As part of an etiological assessment of dyspnea, a transthoracic echocardiography showed a dilated left ventricle, reduced left ventricular ejection fraction at 40%, septo-apical myocardial hypokinesia and left ventricular hypertrabeculation. (Figure 1).
A cardiac magnetic resonance imaging (CMRI) was performed and revealed reduced left ventricular ejection fraction (LVEF) at 30%, globular shape of the left ventricle with an overall wall hypokinesia, hypertrabeculation located at the left ventricle, a thickness of 2.8cm of the trabeculated myocardium on the compact myocardium. less contrast enhancement of the trabeculated myocardium compared to normal, and no thrombus in the left ventricle. The ratio of noncompacted myocardium to compacted myocardium was 3.1. (Figure 2). It was concluded that the appearance was most likely left VNC cardiomyopathy with a LVEF at 30% without intra-cardiac thrombus or mitral insufficiency (Figure 2). Twenty-four hour Holter monitoring showed occasional ventricular premature beats (grade 1 of Lown and Wolf classification)
He was discharged with aspirin 100 mg per day, bisoprolol 2,5 mg per day and Ramipril 2,5 mg per day.
The patient was later lost to follow-up. A few months later, he died without a clear cause explaining the death. A forensic autopsy was performed.
The body was that of a man of average build, with an approximate weight of 70kg. There was no specific sign on the external examination particularly, no asymmetry in comparing the circumference of the two calves.
The heart was globose weighing 325g. The coronary arteries were in a normal position without any significant lesion. The axial dissection of the heart found left ventricle hypertrabeculation with parietal thinning measured at 0.5cm and some intra-cavitary adhering thrombi (Figure 3). Left ventricular examination found wall thickening at 1.2cm. Valvular examination was normal. No systemic thrombi were found. The lungs were the site of profuse oedema. The rest of the organs were congestive without any abnormality. Histologically, the endocardial surface was relatively smooth with anastomosing broad trabeculae resulting in irregular, large staghorn like endocardial lined spaces. A fibrous band separating the spongy from the compact portions of the myocardium was also noted. Toxicology test was negative. Based on clinical history and the necropsy findings, death was attributed to acute ventricle arrhythmia secondary to a myocardium non-compaction.
The European Society of Cardiology (ESC) categorizes VNC as “unclassified cardiomyopathy” with a structural and functional abnormal heart muscle without any other diseases sufficient to cause the observed myocardial abnormality”. However, The American Heart Association (AHA) categorizes it as “genetic cardiomyopathy”4. The etiology and embryogenetic mechanisms leading to VNC are still unknown and several hypotheses are suggested. The most frequent hypothesis is that hypertrabeculation may result from excessive trabeculae formation and/or a defect in the later compaction processes1,5.
VNC, and specifically on the left side, has been found in association with more than 40 mutated genes, which encode for several cell structures. The most common are MYH7, MYBPC3, TTN, at a rate of 71%6. Burke et al.7 reported that the presumed genetic defect resulting in LVNC in the second month of development may cause a variety of other cardiac abnormalities, including epicardial coronary malformation, histiocytoid cardiomyopathy, ventricular septal defects, and conotruncal diseases…In their pathological study of 14 cases of patients with myocardial non compaction, they founded eight patients with associated cardiac anomalies, which did not appear related to the non compaction. They concluded that there was no difference in the gross pattern of trabeculation or microscopic features in the isolated versus the “secondary” forms.
Some cases of VNC associated with congenital hemoglobinopathies have been described in the literature. Kayvanpour E et al.5 have reported cases of LVNC in association with sickle cell disease. Some other cases reported LVNC in a group of family members, including a pair of identical twins, each of whom suffered from thalassemia8.
Although the usual site of hypertrabeculation involvement is the left ventricle, the right ventricle is rarely affected. Right VNC can lead to ventricular tachycardia or right heart failure. In addition, patients with right VNC can be perfectly asymptomatic with only electrocardiographic disorder. In addition, concomitant damage of right VNC is not rare and it can be difficult to distinguish between non-compaction and arrhythmogenic right ventricular cardiomyopathy (ARVD). Diagnosis criteria for ARVD, even if it coexists with typical VNC, may lead to a diagnosis of ARVD rather than VNC. Less frequently, both ventricles can be affected leading to entirely non-compaction cardiomyopathy8.
The most common findings in initial ECGs were left bundle branch block, LV hypertrophy, and repolarisation abnormalities. A normal ECG was rare and rather seen in younger patients with less severe structural cardiac abnormalities9.
Different imaging-based classification systems have been used to make VNC diagnosis. Cardiac Magnetic Resonance Imaging (CMRI)-based criteria (Petersen criteria) is considered as the gold standard10. Not all definitions are anatomically controlled and these criteria are nonspecific. Autopsy performed on individuals with known VNC can be a good way to compare radiological criteria to anatomical findings. Collaboration between forensic medicine and cardiology should be take into consideration with the aim to standardize diagnostic criteria and to avoid over diagnosis in healthy people with a benign prognosis.
Nonspecific histopathological findings have been described, including hypertrophy of the cardiomyocytes, ischemic necrosis with fibrosis due to insufficient vascular supply of the trabeculations, and disorganization of cardiomyocytes11. VNC leads to variable complications that can be misdiagnosed and lead to sudden cardiac death. The most common is conduction defects in approximately 90% of patients followed by myocardium arrhythmia. Thromboembolic events are not very frequent and occur in only 10% of cases, mostly in adults. Three main factors are involved in the occurrence of thromboembolic events: the presence of thrombi into ventricular trabeculations, left ventricular systolic dysfunction with reduced ejection fraction, and/or atrial fibrillation1,3. The prognosis of patients with VNC can vary according to structural and haemodynamic complications. Initial cohorts of VNC suggested 35% to 38% mortality over median 5 to 11 years of follow‐up. However, patients with LVNC with preserved left ventricular ejection fraction had similar survival rate to the general population12.
Currently, there are no guidelines for the management of patients with VNC. Recommendations for treatment include prophylactic anticoagulation therapy and the implantation of a cardiac defibrillator. Treatment for VNC is therefore that of any cardiomyopathy with heart failure13. A periodic check with a 24-hour ECG holter is indicated in order to assess the risk of a possible asymptomatic arrhythmia. Finally, first-degree family members of all patients diagnosed with VNC should undergo an echocardiographic screening examination and genetic exploration14.
In summary, there are multiple controversies related with VNC comprising etiology and pathogenesis, genetic findings, relation with extra-cardiac diseases, diagnostic criteria, treatment, and prognosis. Cardiologists have to pay attention to various clinical manifestations, including heart failure, arrhythmias and cardio embolic events, which can be related to VNC in order to initiate an early treatment and avoiding potentially fatal complications.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication was obtained from the legally authorized representative of the decedent.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular cardiology
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular cardiology
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Grant RT: An unusual anomaly of the coronary vessels in the malformed heart of a child. Heart. 1926; 13: 273-283Competing Interests: No competing interests were disclosed.
Reviewer Expertise: cardiovascular surgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 29 Jul 21 |
read | |
|
Version 1 26 Aug 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)