Keywords
Bone Defect Socket, Carbonate Apatite Scaffold, Medicine, Osteogenic Ability, Stem Cell from Human Exfoliated Deciduous Teeth
Bone Defect Socket, Carbonate Apatite Scaffold, Medicine, Osteogenic Ability, Stem Cell from Human Exfoliated Deciduous Teeth
We have reduced the explanation about periodontitis as a causative factor on the bone defect as we used the tooth extraction induce alveolar bone defect model in rats. We have also added information and explanation regarding experimental animal grouping, control group without treatment SHED in CAS, or SHED group only, CAS role in defective alveolar bone and the study limitation.
See the authors' detailed response to the review by Niswati Fatmah Rosyida
Periodontitis is the second most prevalent oral disease after dental caries1. Approximately 743 million people globally suffer from periodontitis, and this figure has increased by 57.3% over the last ten years2,3. Globally, the losses that are due to reduced productivity caused by severe periodontitis are estimated to be 53.99 million United States (US) dollars annually3,4. Periodontitis is common in Indonesia5. A previous study that was conducted by the Health Ministry of Republic of Indonesia in Basic Health Research (Riset Kesehatan Dasar or RISKESDA) in 2018 showed that there was a 74.1% prevalence of periodontitis6. The rate of periodontitis varies in each country, but together with dental caries, periodontitis is the main reason for tooth loss in adults1,7. Low socio-economic conditions in certain populations will increase the prevalence and extent of the tooth loss, which can result in an alveolar bone defect due to the limited access to dental treatment8. Tooth extraction can lead to alveolar bone resorption and the destruction of the alveolar bone components9. The resorption of the alveolar bone or a reduction in the jawbone dimensions might occur9–11. The presence of periodontal disease, irrational or traumatic dental extraction, periapical root fractures or alveolectomies during dental extractions are considered risk factors for or the etiology of an alveolar bone defect12. An alveolar bone defect can be problematic for dental rehabilitation due to the placement of dental prosthethics13. Osseointegrated of dental implants with sufficient initial stability requires adequate bone quality and quantity. Moreover, it is suggested that socket preservation is performed to enhance the success of the osseointegrated dental implants14.
In the dental medicine field, the management and rehabilitation of alveolar bone defects has long been viewed as a challange15. To overcome alveolar bone defects, dentists must consider bone grafting surgeries for socket preservation to obtain an adequate bone density, volume, quality, and geometry for the implant placement. This will enable osseointegration of the dental implant15.
There have been many attempts to overcome alveolar bone defects, such as bone grafts, platelet rich fibrin (PRF), mesenchymal stem cells, hematopoetic stem cells, and herbal medicine16–23. Bone grafts are still not effective; therefore, alternative tissue engineering approaches are required24.
The current most promising treatment for an alveolar bone defect is through regenerative medicine, which uses tissue engineering. This tissue engineering involves three components, and is therefore referred to as triad tissue engineering: growth factors, stem cells, and a scaffold25,26. Mesenchymal stem cells (MSCs) can differentiate into various cells, such as osteogenic, adipogenic and chondrogenic differentiations27. The oral cavity provides a rich source of MSCs. MSCs, such as gingiva mesenchymal stem cells (GMSCs), dental pulp stem cells (DPSCs), and stem cells from human exfoliated deciduous teeth (SHED), can be easily isolated and obtained from the oral cavity tissue using minimally invasive procedures comparted to those needed for bone marrow mesenchymal stem cells (BM-MSCs)28–31.
SHED is one of the MSCs from the oral cavity that can be used to regenerate damaged tissue, such as an alveolar bone defect24. SHED is capable of differentiating and proliferating. Moreover, to optimally facilitate SHED proliferation, cell growth, and differentiation, a biocompatible cell carrier or scaffold is necessary32. Carbonate apatite is a biomaterial that is commonly used as a scaffold. Carbonate apatite has been clinically proven to be a good bone scaffold for the regenerative medicine33. The study about combination of SHED and CAS ameliorate alveolar bone defect post tooth extraction is still limited. The hypothesis of this study is that the number of osteogenic markers expressing cells, such as OPG, RUNX2, TGF-β, VEGF, ALP, osteocalcin, and ostepontin, would increase in the alveolar bone defects seven days after being implanted with SHED seeded in carbonate apatite scaffold (CAS), with the exception of the receptor activator of NF-κβ ligand (RANKL) expression. Osteocalcin, osteopontin, ALP, RUNX are the osteogenic differentiation markers of SHED. CAS can facilitate the osteogenic differentiation of SHED in vitro. Meanwhile, RANKL / OPG ratio are well-known as markers that can be used to predict the success of bone remodeling. Some growth factors are secreted by SHED, such as TGF-β and VEGF, which have an important role in supporting bone formation and controlling the inflammation process17–24,32–34. Wistar rats (Rattus novergicus) were selected as the animal models because many studies have used this animal to study the effect of medication on the alveolar bone defects21–24. Additionally, these rats are not aggressive, and they are easy to handle and observe. This made them suitable animal models to induce the response of the human tissue system. Furthermore, the purpose of this study is to examine the ability of SHED and CAS in the alveolar bone defects using an immunohistochemical analysis.
All experimental procedures involving animals were carried out in accordance with the guidelines from the National Health Institute on the care and use of laboratory animals to ameliorate any suffering for the animals.
This study was an experimental laboratory design. A post-test-only control group study design was conducted. The formulation that used to calculate the sample size in this study was sample size = 2SD2(Zα/2+ Zβ)2/d2 where the standard deviation (SD) = 1.1; Zα/2 = Z 0.05/2 = Z0.025 =1.96 (from Z table) at type 1 error of 5%; Zβ = Z0.20 = 0.842 (from Z table) at 80% power; d = effect size = 1.94. The number of samples, which was five trial animals in each group. The sample in each group was randomly chosen by giving each trial animal a tag number. Following that, the researcher randomly chose the tag numbers.
The SHED were obtained from deciduous teeth using the following criteria: #83, #73 deciduous tooth, free of caries, no root resorption, and a vital and intact pulp was obtained through tooth extraction from a healthy, 7–10 years-old pediatric patient who underwent orthodontics treatment. The healthy deciduous tooth was extracted from healthy pediatric patients undergoing orthodontics treatment performed at the Dental Hospital, Universitas Airlangga, Surabaya, Indonesia. Patient anonymity was maintained and written informed consent was obtained from the patient’s parents. Ethical approval was obtained from the Universitas Airlangga, Faculty of Dental Medicine ethics committee (171/HRECC.FODM/VIII/2017) that covered for both human sampling and the animal procedures.
The dental pulp cavity was opened using drills under aseptic condition. The dental pulp was isolated with a broach then washed three times with phosphate-buffered saline (PBS). Dental pulp tissue was minced into small pieces (≤0.5 mm) in 10-cm culture dishes digested in a solution of 3 mg/mL collagenase type I (no cat. CLS-01, Worthington Biochem, Freehold, NJ) and 4 mg/mL Dispase® II (cat no. 42613-33-2, Sigma Aldrich, USA) for 1 h at 37°C. Dulbecco′s Modified Eagle′s Medium (cat no. D5030, Merck, US), was utilized to culture the dental pulp from the deciduous tooth. Fetal bovine serum (FBS, catcat no. F2442, Merck, US) with 20% concentration, five milimeter L-glutamine (cat no. 5030081, Gibco Invitrogen®, 25, USA), 100 U/ ml penicillin-G, 100 ug/ml streptomycin, and 100 ug/ml kanamycin (cat no. 15140163, Gibco Invitrogen®, 25, USA) was added34.
Every four days, the medium was changed to eliminate the unattached cell on the culture plate and the cells were maintained up to four passages. Phosphate Buffer Saline was used to wash the cells to eliminate debris. Trypsin-EDTA 0.05% was applied to detach the cells and transfer them onto a bigger culture plate. After the cells reached 70–80% confluence was obtained, the SHED cells in the 4 passaged were then prepared for the next step of the study24,32,34.
Ten three-month-old healthy male Wistar rats (R. novergicus) that weighed between 150–250 grams (g) were used as animal models and were obtained from the Research Center of Faculty of Dental Medicine, Universitas Airlangga (UNAIR) Surabaya, Indonesia. Ten wistar rats were assigned into two groups respectively; CAS group and CAS+SHED group.
All experimental procedures involving animals were carried out in keeping with guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals to ameliorate any suffering of animals35. The animal models were acclimatized for a week at a temperature of 21–23 °C with controlled humidity (50 ± 5%) in a 12-hour artificial light cycle (8 am to 8 pm) to help them to adapt to the same conditions, as they had various origins. All the rats were located individually in polycarbonate cages (0.90 × 0.60 × 0.60 m). Furthermore, every animal model was fed with standard pellet, and water was provided ad libitum with the husk replaced every three days. All animal models were routinely inspected and observed regarding their food consumption and fecal characteristics20.
Rodent anesthesia of 0.1 mL/10 grams body weight (BW) (160095, Kepro™, Netherlands), and xylazine (160096, Xyla™, Netherlands) (ketamine dose 35 mg/kg body weight and xylazine five mg/kg body weight) was administered intramuscularly on the gluteus muscle to ameliorate the pain during the procedure of inducing the alveolar bone defects on the animal models. Sterile needle holder clamps were used to extract the anterior teeth of the mandibular to induce the alveolar bone defects in the animal models36.
After the alveolar bone defect was induced, the transplantation of the SHED seeded in CAS or CAS only was performed in the afflicted area. Before being placed in a 24-well tissue culture plate and prepared for the experimental group, a 20 ml suspension of SHED at passage four to five with a density of 106 cells was seeded into CAS (no cat AKD 20602410125, GAMACHA, Swayasa Prakarsa Company, Indonesia). The dose was determined based on the evidence from a previous in vivo study, which was 106 cells per sample34. To perform the interrupted suture to fix the wound after transplantation, a 5.0 suture monofilament was used24,32,34.
Seven days post transplantation, all the animal models were terminated using an overdosed rodent anesthesia with an intravenous injection of 100 mg/kg BW (Pentobarbital, 1507002, Pubchem, USA). We used this euthanasia method to ameliorate animal suffering that arises from the termination process. After the termination of animal study, we collected the afflicted alveolar bone samples for further histological analysis. The animal model’s head was cut from the back by sterile sharp surgical scissors (metzenbaum scissors fine tips, no cat. 3565, Medesy, Maniago, Italy) and tweezer (Tweezer de bakey mini, no cat. 1007/10-TO, Medesy, Maniago, Italy), exposing the anterior of the mandible allowing the afflicted alveolar bone sample to be obtained. Before sample collection, all the animals were observed for any general toxicity probability, including edema or death, and measured had their body weight (using a digital scale, ZB22-P, Zieis®, USA). All these measurements were done by a single blinded observer. The afflicted tissue was then extracted and immersed in 10% neutral buffer formalin for fixation.
The sample was decalcified and immersed in 10% EDTA (cat no. 17892, Ajax Finechem, Thermo Fisher Scientific; Taren Point, Australia). Following that, the samples were underwent tissue processing overnight (Leica TP1020, USA), prior to embedding in molten paraffin wax (HistoCore Arcadia H - Heated Paraffin Embedding Station, Leica, USA). Sections were cut at 5 µm rotary microtome (RM2235, Leica, USA). Paraffin ribbons were flattened in a water bath at 40°C and collected onto polysine microscope slides (Thermo Scientific) prior to drying at 60°C for 16 hr (Sakura Heater, Tokyo, Japan)37.
Immunohistochemistry staining was conducted using a 3.3'-diaminobenzidine stain kit (DAB) (cat noD7304-1SET, Sigma Aldrich, US). Antibody monoclonal (AbMo) of RANKL 1:500 dilution (cat. no sc-377079), osteoprotegrin (OPG) 1:500 dilution (cat. no sc-390518), runt-related transcription factor 2 (RUNX2) 1:500 dilution (cat. no sc-390351), transforming growth factor-β (TGF-β) 1:500 dilution (cat. no sc-130348), vascular endothelial growth factor (VEGF) 1:500 dilution (cat. no sc-7269), alkaline phosphatase (ALP) 1:500 dilution (cat. no sc-271431), osteocalcin (cat. no sc-365797)) 1:500 dilution, and ostepontin (cat. no sc-21742) 1:500 dilution were used in this study (Santa Cruz Biotechnology™, US). The observation and examination of the number of the RANKL, OPG, RUNX2, TGF-β, VEGF, ALP, osteocalcin, and ostepontin expressions in the periodontal tissue were performed manually by two observers in five perspective fields of view by utilizing Nikon H600L light microscope (Japan) at 400x magnification. We also provide 200x and 1000x magnification of each marker for context (Nikon, Japan)37.
The Statistical Package for Social Science (SPSS) 20.0 version (IBM corporation, Illinois, Chicago, United State) software was used in this study to analyze the data. To compare the significant differences between the groups in the RANKL, OPG, RUNX2, TGF-β, VEGF, ALP, osteocalcin, and ostepontin expressions, a t-test was employed (p<0.01). The OPG and RANKL expressions’ association was examined using Pearson’s correlation test (p<0.05).
The transplantation of SHED seeded in CAS or CAS only at selected doses did not lead to any general toxicity, edema, death or changes in body weight of the rats (see underlying data38). The expressions of OPG, RUNX2, TGF- β, VEGF, ALP, osteocalcin, and osteopontin in SHED seeded in CAS were greater than in the CAS only group. In comparison, the RANKL expression was lower in SHED seeded in CAS compared to CAS only (see Figure 1–Figure 439–46). There was a significant increase in OPG, RUNX2, TGF-β VEGF, ALP, osteocalcin, and osteopontin expressions and decreased RANKL expression in SHED seeded in CAS compared to CAS only (p<0.01). There was a significant strong reverse correlation between the OPG and RANKL expressions (p<0.01) (Table 1).

Immunohistochemistry with antibody monoclonal (AbMo) and DAB were performed to examine the (A) RANKL and (B) OPG expressions. The positive cells were stained brown (black box) with a 200x, 400x, and 1000x magnification using a light microscope. The number of osteoblasts expressing (C) RANKL and (D) OPG in the alveolar bone of the rats.
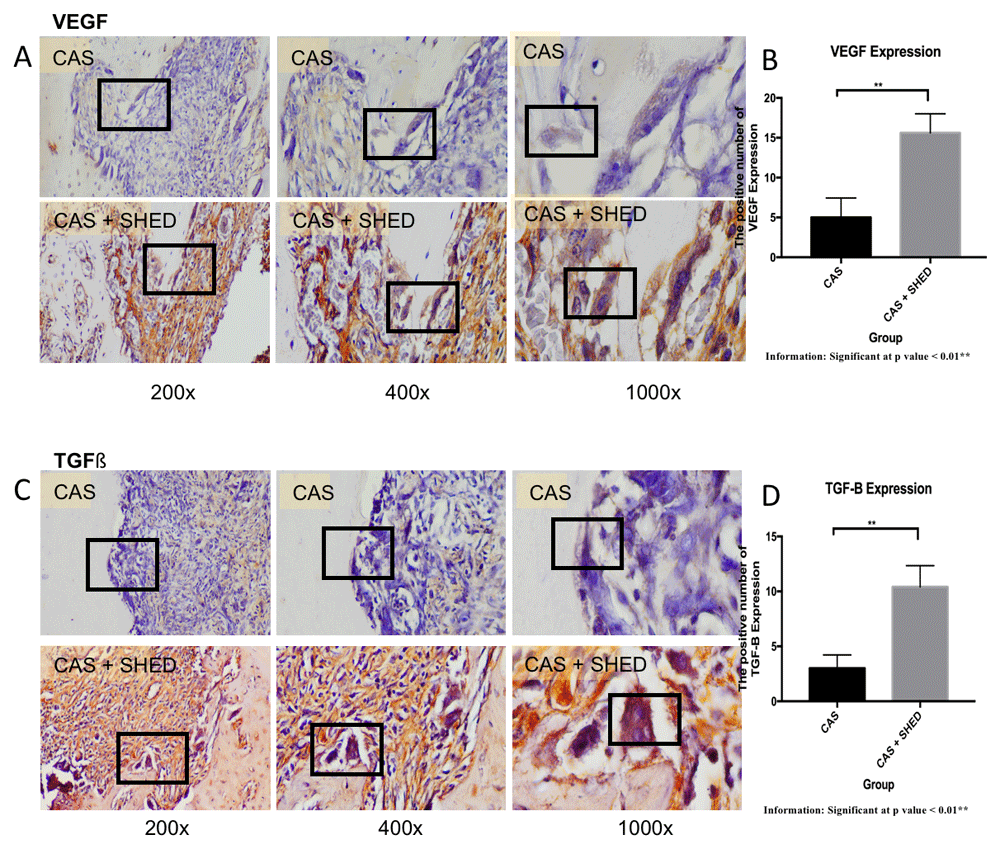
Immunohistochemistry with antibody monoclonal (AbMo) and DAB were performed to examine the (A) VEGF and (B) TGF-β expressions. The positive cells were stained brown (black box) with 200x, 400x, and 1000x magnification using the light microscope. The number of osteoblasts expressing (C) VEGF and (D) TGF- β in the alveolar bone of the rats.

Immunohistochemistry with antibody monoclonal (AbMo) and DAB were performed to examine the (A) RUNX2 and (B) ALP expressions. The positive cells were stained brown (black box) with 200x, 400x, and 1000x magnification using the light microscope. The number of osteoblasts expressing (C) RUNX2 and (D) ALP in the alveolar bone of the rats.

Immunohistochemistry with antibody monoclonal (AbMo) and DAB were performed to examine the (A) osteocalcin and (B) osteopontin expressions. Positive cells were stained brown (black box) in 200x, 400x, 1000x magnification by using the light microscope. The number of osteoblasts expressing (C) osteocalcin and (D) osteopontin in the alveolar bone of the rats.
| Group | Molecular Marker | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± Standard Deviation | ||||||||
| OPG | RANKL | TGF- β | VEGF | RUNX2 | ALP | OSC | OSP | |
| CHA | 5.4 ± 0.6 | 4.8 ± 0.5831 | 3 ± 0.5477 | 5 ± 1.095 | 4.4 ± 0.6782 | 10.4 ± 2.073 | 7.64 ± 0.6 | 3.8 ± 0.3742 |
| *Normality | 0.21 | 0.421 | 0.146 | 0.146 | 0.86 | 0.23 | 0.201 | 0.314 |
| CHA+SHED | 11.6 ± 1.077 | 12.2 ± 0.5831 | 10.4 ± 0.8718 | 15.6 ± 1.077 | 13.4 ± 1.077 | 18.4 ± 1.949 | 13.2 ± 1.281 | 13 ± 1.14 |
| *Normality | 0.787 | 0.21 | 0.758 | 0.787 | 0.787 | 0.758 | 0.823 | 0.207 |
| **Sig | 0.001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0042 | 0.0001 |
| Pearson Correlation | -0.0801 | |||||||
| **Sig. Correlation | 0.005 | |||||||
Information: *significant at p value > 0.05; **significant at p value < 0.01. RANKL - receptor activator of NF-κβ ligand, OPG - osteoprotegrin, TGF- β - transforming growth factor-β, VEGF - vascular endothelial growth factor, RUNX2 - runt-related, transcription factor 2, ALP - alkaline phosphatase, OSC - osteocalcin, OSP osteopontin, SHED- stem cells from human exfoliated deciduous teeth, CHA - carbonate hydroxyapatite
Severe alveolar defect has become a problem for both the patients and clinicians, especially regarding dental implant placement and ossteointegration15.
This experimental study confirms the hypothesis that the transplantation of SHED seeded in CAS could increase the number of osteogenic markers expressing cells, such as OPG, RUNX2, TGF-β, VEGF, ALP, osteocalcin, and ostepontin, but not the RANKL expression in the bone defects after seven days in comparison to the CAS group17–20,22,32.
This result supports the theory that SHED possess functions that can enhance OPG to bind to RANKL, which results in the inhibited osteoclastogenesis34. There is a strong reverse significant correlation between OPG and RANKL expressions in this study. The SHED with the scaffold increases the OPG expression meanwhile, decreases the RANKL expression, which is supported by the previous study by Prahasanti et al.34
CAS plays an important role in supporting SHED proliferation and differentiation24,32. RUNX2, ALP, osteocalcin, and osteopontin are osteogenic differentiation markers of MSCs. These markers are essential and important for the analysis of osteoblastogenesis and bone regeneration17,18,20. ALP expression increases due to the signaling bone morphogenic protein (BMP), RUNX2, osterix system, and Wnt cascade interacting with each other. The increased expression of RUNX can enhance ALP expression17,18. Several growth factors also stimulate the activation of the MSCs’ osteogenic differentiation, such as VEGF and TGF- β. TGF-β significantly increases the expression of the early-phase osteogenic differentiation marker genes47. VEGF is associated with all the bone formation steps, including mesenchymal condensation48. VEGF has a direct influence on the MSC osteogenic differentiation through the regulation of osteoprogenitors using the angiocrine function. VEGF recruits immune cells to the osteogenic niche49.
The osteogenic microenvironment in defective alveolar bone can induce SHED to differentiate into bone cells, especially osteoblast24,32,34. The activation of the osteorix and RUNX2 systems can stimulate the expression of osteocalcin and osteopontin17. OSC is a secreted protein that is dependent on Vitamin K, a macromolecule with a role in bone mineralization18. Osteopontin plays a pivotal role in bone remodeling, regulating osteoclastogenesis, osteoclast activity, and differentiation. Osteopontin maintains the bone mineral matrix inorganic components of bone, such as hydroxyapatite, Ca(PO4)(OH)2. Osteopontin, which is expressed in osteoblasts, is responsible for bone remodeling in bone homeostasis20. Both osteocalcin and osteopontin are important for bone maturation because they are major non-collagenous proteins that are involved in bone matrix organization and deposition.
Osteocalcin and osteopontin are produced during bone formation49. Both of them control––either directly and/or indirectly–the mass, mineral size, and orientation50–52. Both proteins also play structural roles in the bone and determine the bone’s propensity to fracture53. This is in accordance with our findings, as it states that there is a significant enhancement of the OPG, RUNX2, TGF- β, VEGF, ALP, osteocalcin, and osteopontin expressions, and the decreased RANKL expression is more significant in Group II than Group I. Bone regeneration is a complex process that requires highly orchestrated interactions between different cells and signals to form the new mineralized tissue54. MSCs have the ability to differentiate into osteoprogenitors and osteoblasts, as well as to form the calcified bone matrix55.
SHED have the potential to play a significant role in tissue engineering and regenerative medicine. A previous study by Nakajima et al. declared that SHED, in comparison to the hDPSCs or hBMSCs group, produce the largest osteoid and collagen fibers. Furthermore, SHED transplantation possess a potential and sufficient ability for bone regeneration to repair the bone defect56,57.
The limitations of this study were that the observation and evaluation were performed seven days post transplantation of SHED seeded in CAS on the animal model, and only an immunohistochemical examination was performed. Further studies will be necessary to evaluate the changes in the alveolar bone and periodontal tissue post transplantation of SHED seeded in CAS in the alveolar bone defect animal models. With a longer observation time, further studies using methods, such as qRT-PCR and/or the western blot analysis, could be conducted to estimate the expression of bone molecular markers. Future studies are also required to confirm the effective dose of the used biomaterials when it is ready to be applied in the clinical study of humans.
In conclusion, the expression of OPG, RUNX2, TGF-β, VEGF, ALP, osteocalcin, and ostepontin increases significantly with treatment with SHED seeded in CAS. Moreover, the RANKL expression in the alveolar bone defect did not increase in SHED seeded in CAS as documented immunohistochemically.
Figshare: RANKL. https://doi.org/10.6084/m9.figshare.12609986.v139
This project contains the following underlying data:
= CAS RANKL 200x.jpg (Expression of RANKL at 200x magnification in the CAS group)
= CAS RANKL 400x.jpg (Expression of RANKL at 400x magnification in the CAS group)
= CAS RANKL 1000x.jpg (Expression of RANKL at 1000x magnification in the CAS group)
= CAS SHED RANKL 200x.jpg (Expression of RANKL at 200x magnification in the CAS SHED group)
= CAS SHED RANKL 400x.jpg (Expression of RANKL at 400x magnification in the CAS SHED group)
= CAS SHED RANKL 1000x.jpg (Expression of RANKL at 1000x magnification in the CAS SHED group)
Figshare: OPG. https://doi.org/10.6084/m9.figshare.12609983.v140
This project contains the following underlying data:
= CAS OPG 200x.jpg (Expression of OPG at 200x magnification in the CAS group)
= CAS OPG 400x.jpg (Expression of OPG at 400x magnification in the CAS group)
= CAS OPG 1000x.jpg (Expression of OPG at 1000x magnification in the CAS group)
= CAS SHED OPG 200x.jpg (Expression of OPG at 200x magnification in the CAS SHED group)
= CAS SHED OPG 400x.jpg (Expression of OPG at 400x magnification in the CAS SHED group)
= CAS SHED OPG 1000x.jpg (Expression of OPG at 1000x magnification in the CAS SHED group)
Figshare: RUNX2. https://doi.org/10.6084/m9.figshare.12610478.v141
This project contains the following underlying data:
= CAS RUNX2 200x.jpg (Expression of RUNX2 at 200x magnification in the CAS group)
= CAS RUNX2 400x.jpg (Expression of RUNX2 at 400x magnification in the CAS group)
= CAS RUNX2 1000x.jpg (Expression of RUNX2 at 1000x magnification in the CAS group)
= CAS SHED RUNX2 200x.jpg (Expression of RUNX2 at 200x magnification in the CAS SHED group)
= CAS SHED RUNX2 400x.jpg (Expression of RUNX2 at 400x magnification in the CAS SHED group)
= CAS SHED RUNX2 1000x.jpg (Expression of RUNX2 at 1000x magnification in the CAS SHED group)
Figshare: TGF-Beta. https://doi.org/10.6084/m9.figshare.12610487.v142
This project contains the following underlying data:
= CAS TGF-β 200x.jpg (Expression of TGF-β at 200x magnification in the CAS group)
= CAS TGF-β 400x.jpg (Expression of TGF-β at 400x magnification in the CAS group)
= CAS TGF-β 1000x.jpg (Expression of TGF-β at 1000x magnification in the CAS group)
= CAS SHED TGF-β 200x.jpg (Expression of TGF-β at 200x magnification in the CAS SHED group)
= CAS SHED TGF-β 400x.jpg (Expression of TGF-β at 400x magnification in the CAS SHED group)
= CAS SHED TGF-β 1000x.jpg (Expression of TGF-β at 1000x magnification in the CAS SHED group)
Figshare: VEGF. https://doi.org/10.6084/m9.figshare.12610484.v143
This project contains the following underlying data:
= CAS VEGF 200x.jpg (Expression of VEGF at 200x magnification in the CAS group)
= CAS VEGF 400x.jpg (Expression of VEGF at 400x magnification in the CAS group)
= CAS VEGF 1000x.jpg (Expression of VEGF at 1000x magnification in the CAS group)
= CAS SHED VEGF 200x.jpg (Expression of VEGF at 200x magnification in the CAS SHED group)
= CAS SHED VEGF 400x.jpg (Expression of VEGF at 400x magnification in the CAS SHED group)
= CAS SHED VEGF 1000x.jpg (Expression of VEGF at 1000x magnification in the CAS SHED group)
Figshare: ALP. https://doi.org/10.6084/m9.figshare.12610493.v144
This project contains the following underlying data:
= CAS ALP 200x.jpg (Expression of ALP at 200x magnification in the CAS group)
= CAS ALP 400x.jpg (Expression of ALP at 400x magnification in the CAS group)
= CAS ALP 1000x.jpg (Expression of ALP at 1000x magnification in the CAS group)
= CAS SHED ALP 200x.jpg (Expression of ALP at 200x magnification in the CAS SHED group)
= CAS SHED ALP 400x.jpg (Expression of ALP at 400x magnification in the CAS SHED group)
= CAS SHED ALP1000x.jpg (Expression of ALP at 1000x magnification in the CAS SHED group)
Figshare: Osteocalcin. https://doi.org/10.6084/m9.figshare.12610481.v145
This project contains the following underlying data:
= CAS osteocalcin 200x.jpg (Expression of osteocalcin at 200x magnification in the CAS group)
= CAS osteocalcin 400x.jpg (Expression of osteocalcin at 400x magnification in the CAS group)
= CAS osteocalcin 1000x.jpg (Expression of osteocalcin at 1000x magnification in the CAS group)
= CAS SHED osteocalcin 200x.jpg (Expression of osteocalcin at 200x magnification in the CAS SHED group)
= CAS SHED osteocalcin 400x.jpg (Expression of osteocalcin at 400x magnification in the CAS SHED group)
= CAS SHED osteocalcin 1000x.jpg (Expression of osteocalcin at 1000x magnification in the CAS SHED group)
Figshare: Ostepontin. https://doi.org/10.6084/m9.figshare.12610490.v146
This project contains the following underlying data:
= CAS osteopontin 200x.jpg (Expression of osteopontin at 200x magnification in the CAS group)
= CAS osteopontin 400x.jpg (Expression of osteopontin at 400x magnification in the CAS group)
= CAS osteopontin 1000x.jpg (Expression of osteopontin at 1000x magnification in the CAS group)
= CAS SHED osteopontin 200x.jpg (Expression of osteopontin at 200x magnification in the CAS SHED group)
= CAS SHED osteopontin 400x.jpg (Expression of osteopontin at 400x magnification in the CAS SHED group)
= CAS SHED osteopontin 1000x.jpg (Expression of osteopontin at 1000x magnification in the CAS SHED group)
Figshare: Raw Data Bone Molecular Markers. https://doi.org/10.6084/m9.figshare.12610499.v158
This project contains the following underlying data:
= Raw Data Molecular Marker.xlsx (The raw data of molecular markers examined by means of IHC analysis)
Figshare: Animal Body Weight. https://doi.org/10.6084/m9.figshare.12610502.v138
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomaterial in Orthodontic, Biological Tooth Movement
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomaterial in Orthodontic, Biological Tooth Movement
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 02 Dec 20 |
read | |
|
Version 1 22 Sep 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)