Keywords
Immunotherapy, checkpoint inhibitors, pembrolizumab, malignant melanoma, multiple sclerosis, malignancy, antigens, toxicites, adverse effects, steroids
Immunotherapy, checkpoint inhibitors, pembrolizumab, malignant melanoma, multiple sclerosis, malignancy, antigens, toxicites, adverse effects, steroids
Immune checkpoint inhibitor therapy has transformed the cancer care landscape1. It is being used to treat various malignancies with significant improvement in survival noted in solid tumors such as melanoma and non-small cell lung carcinoma, etc2. This has been an attractive alternative for many oncologists given its efficacy and minimal toxicity across the spectrum of program death ligand-1 (PDL-1) and program death-1 (PD-1) inhibitors3. However, they result in unique toxicities related to immune modulation and activation such as immune-mediated endocrinopathies such as hypothyroidism, pneumonitis, colitis, hepatitis which are seemingly more common compared to rare complications such as myocarditis, meningitis, hypophysitis, etc4. As such, checkpoint inhibitor therapy is contraindicated in patients with autoimmune disorders or recipients of organ transplants given the risk for reactivation or flare of the underlying autoimmune disease and rejection of the donor organ in transplants, although sporadic cases have been reported with the use of immunotherapy in such patients5. In this report, we present a case of checkpoint inhibitor therapy in a patient with multiple sclerosis (MS) who underwent immune checkpoint inhibitor therapy with pembrolizumab for metastatic malignant melanoma.
A 73-year-old female with a past medical history of MS with chronic right hemi-somal deficits on baclofen (10 mg twice daily), paroxetine (40 mg daily), and gabapentin (300 mg three times daily). She had prior history of cutaneous malignant melanoma (MM) in 2015 post excision and negative sentinel lymph nodes noticed left inguinal swelling before November in 2018 and a PET scan revealed prominent and bulky metabolic adenopathy involving the left inguinal, external iliac, proximal internal iliac regions, and common iliac region lymphadenopathy (Figure 1 and Figure 2). Left inguinal mass biopsy revealed metastatic malignant melanoma (Figure 3 and Figure 4). Tissue from the biopsy tested positive for HMB-45 and Melan-A antibodies (Figure 5 and Figure 6). Biomarker testing revealed no evidence of BRAF or KIT mutations; TERT, NRAS mutations and MDM2 copy gain was present, MSI-stable, and the TMB high at 8.8 mutation/MB (87th percentile). Magnetic resonance imaging (MRI) of the brain was negative for metastatic malignancy however showed non-specific white matter changes consistent with demyelinating plaques linked to her history of multiple sclerosis without finding of active disease (Figure 7 and Figure 8).
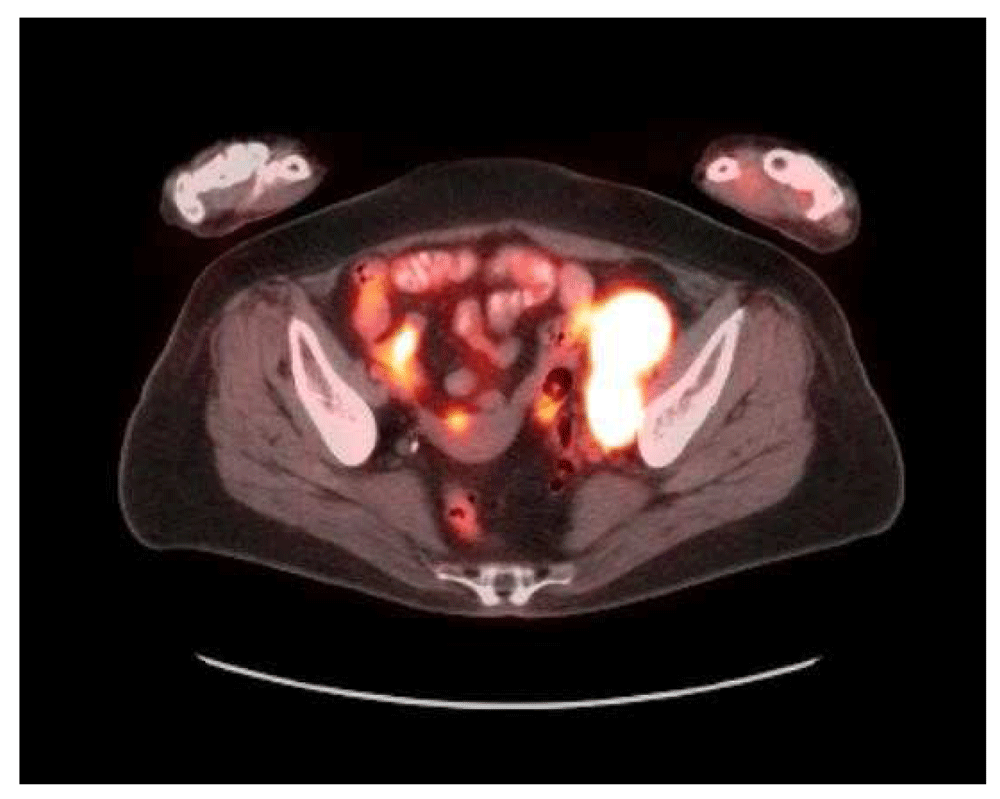
Multiple prominently metabolic nodes are visualized throughout the left external iliac, proximal internal iliac and common iliac regions. 1 of the more prominent nodes situated in the distal external iliac chain spanning 2.8 × 4.2 cm with an SUV max of 18.4 on image 250. Small metabolic nodes are also visualized in the lower retroperitoneum up to the L4 level.
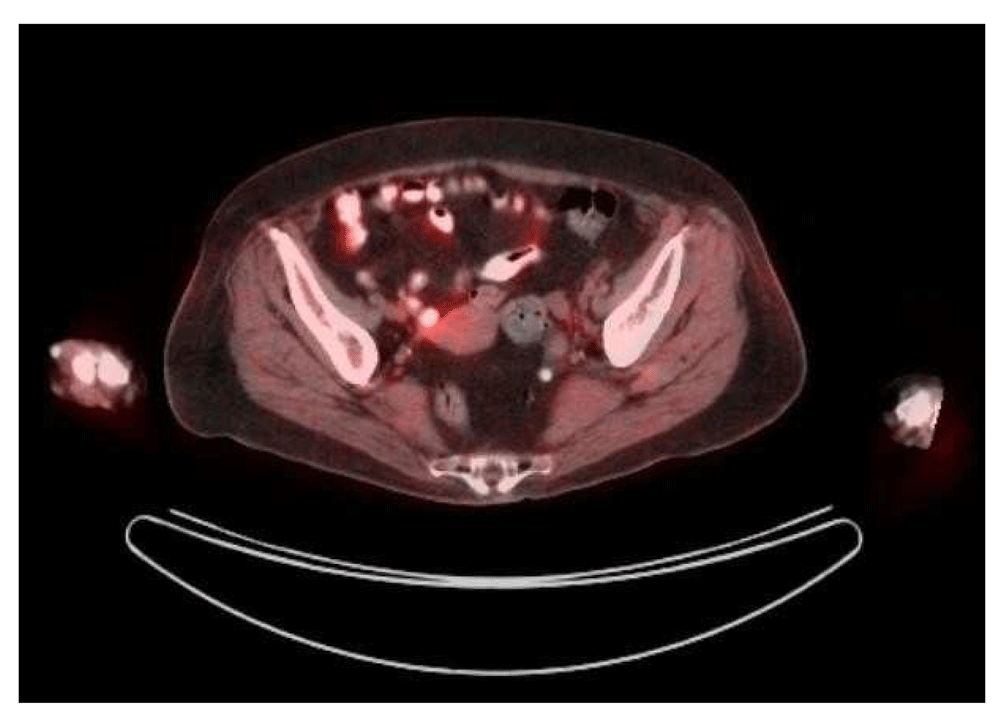
For example, a left inguinal node is 1.3 × 1.8 cm with an SUV max of 1.0; previously a nodal conglomerate in this region was 2.8 × 5.6 cm and SUV max 21.1. A small residual nodal prominence in the left external iliac region is 0.9 × 2.7 cm, SUV max 2.0; previously 2 adjacent nodes in this region or 3.3 × 7.6 cm and SUV max 19.1. No new metabolic nodal lesions are visualized in the inguinal regions, pelvis or throughout the abdomen
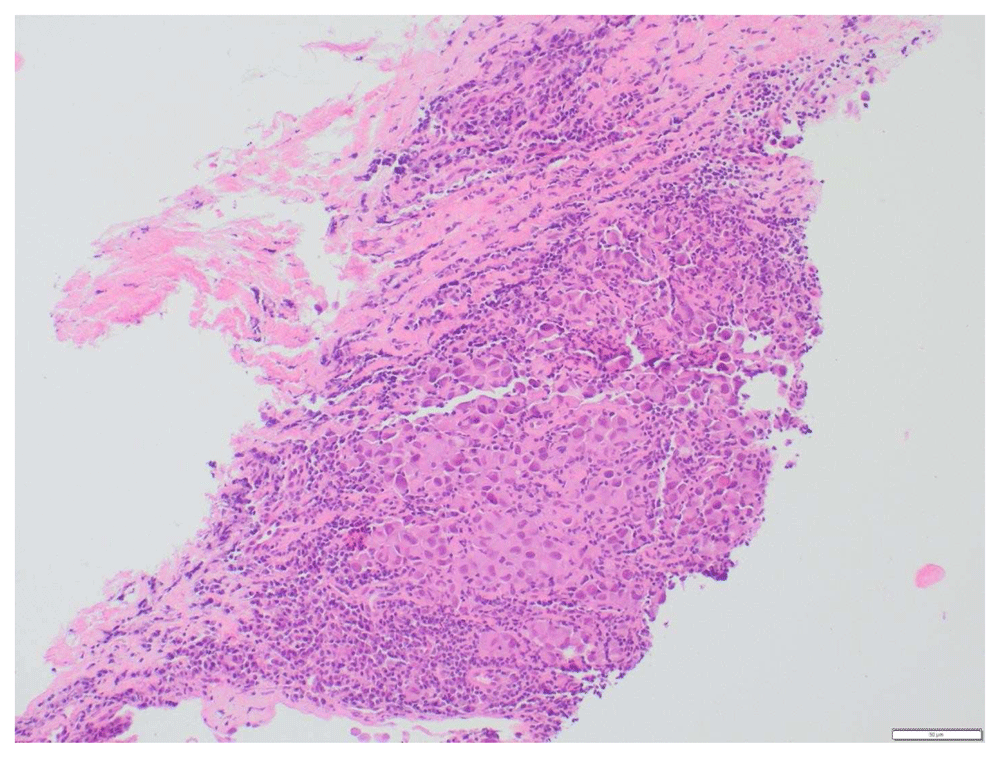
In the background you can see some normal lymphocytes (smaller dark blue staining cells). Magnification, ×20.
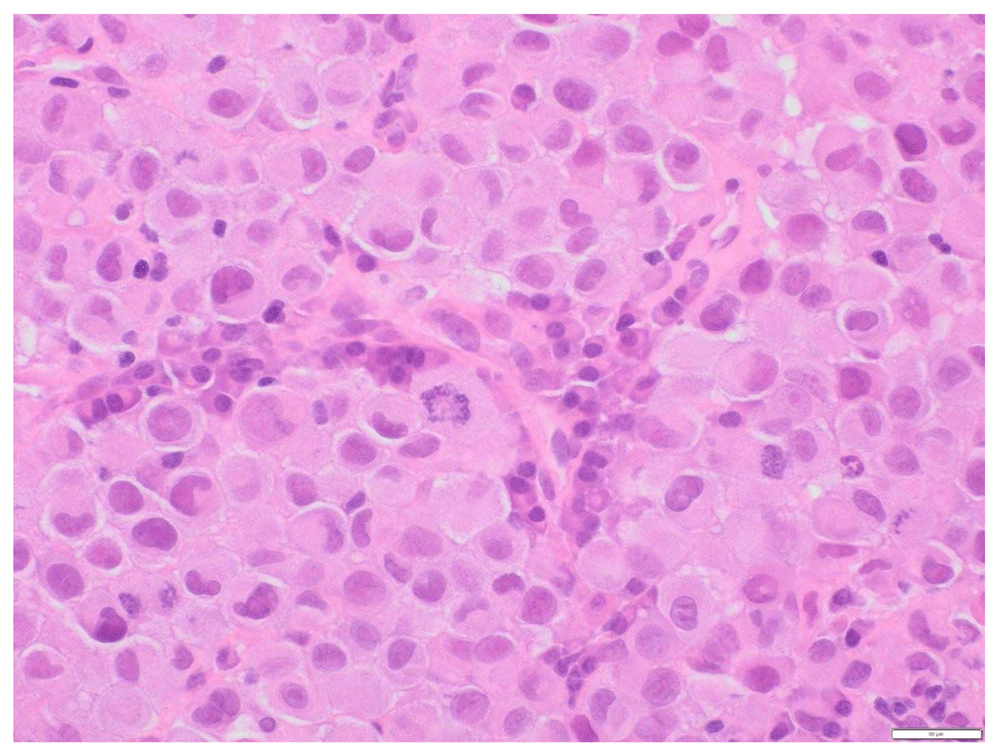
There are few abnormal mitotic figures and some plasma cells in the background.
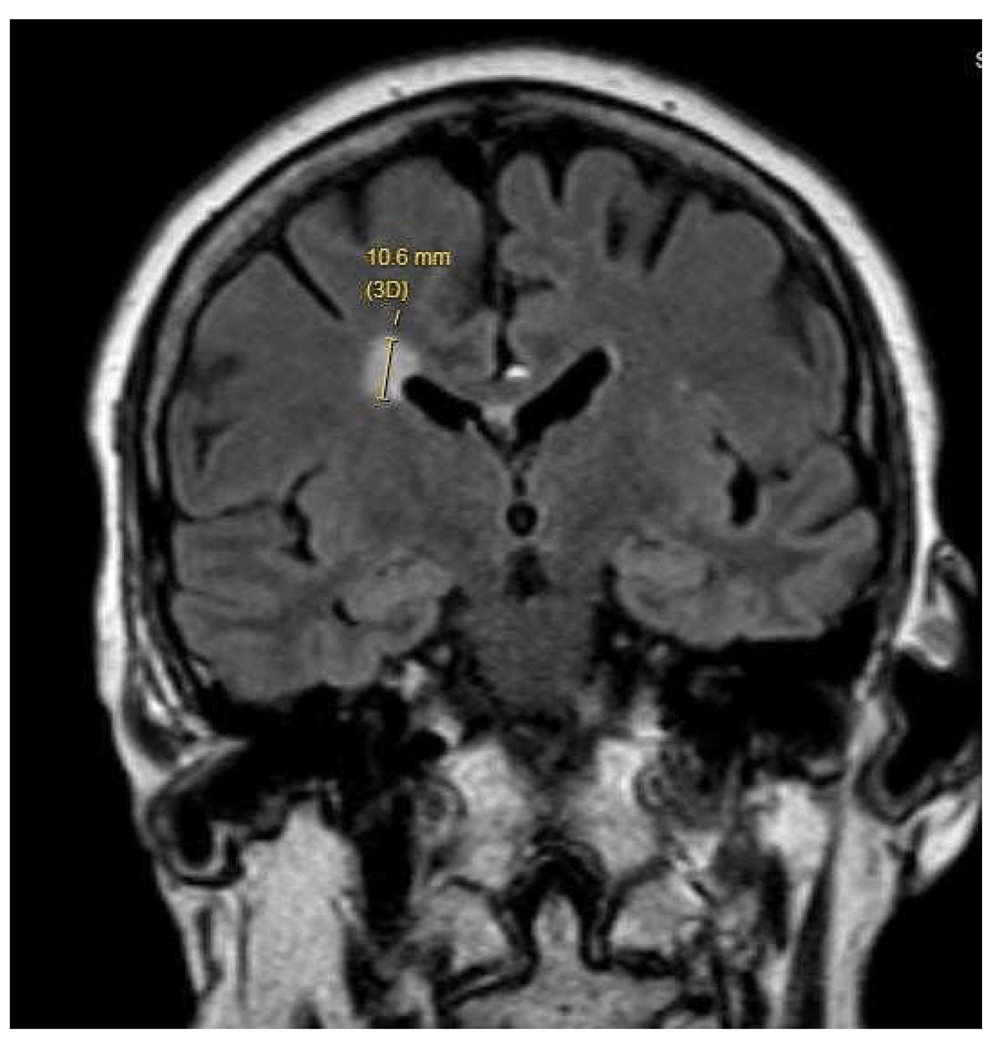
Nonspecific focal white matter lesions could reflect multiple sclerosis, without finding of active disease.
After discussing the pros and cons related to immune checkpoint inhibitors (ICI) versus salvage chemotherapy; she expressed understanding of the potential but significant risk of MS flare-up. She was initiated on pembrolizumab 200 mg every 3 weeks at 4 weeks after diagnosis. Unfortunately, two weeks after the first cycle of pembrolizumab therapy she encountered significant lethargy, slurred speech, word-finding difficulty, stiffness and pain in her bilateral groins, gait imbalance with recurrent falls, generalized weakness requiring assistance for bed transfer. Pertinent physical exam findings included pupils that were reactive to light both directly and consensually; the fundoscopic exam was normal with the cup to disc ration, no edema/hemorrhages; apraxia, tremors in bilateral hands; decreased motor strength (4/5 using the Oxford scale) in the bilateral upper and lower extremity muscle groups; deep tendon reflexes 2/4 in the left and 3/4 in the right biceps, triceps, patellar and achilles. Hoffman, Tromner's, and Babinski signs were present on the right; sustained clonus was noted in right ankle and ataxia was present. Complete blood picture, electrolytes, kidney, and liver function were essentially unremarkable. Her signs and symptoms were concerning for an MS flare-up, and an MRI of the brain with and without contrast revealed enhancing white matter lesion on the right external capsule, larger in size and hyperintense per the gadolinium contrast; EEG was unremarkable. In light of clinical and radiological findings consistent with an MS flare-up, she was started on prednisone 1 mg/kg with a prolonged taper over 8 weeks by 10 mg every week with subsequent significant improvement in treatment-related neurological symptoms in the coming weeks with active participation in physical therapy (PT).
She had an interval improvement in the tumor size with just one round of treatment, which has been correlated in studies with patients encountering immune-related adverse effects (iAE’s) often having better responses than their counterparts who do not have iAE’s6. CT imaging of the abdomen/pelvis with contrast imaging post-cycle showed significant interval response with the adenopathy decreased by >50%, for example, left inguinal lymph node decreased from 4.6 to 1.3 cm, left external iliac adenopathy decreased from 4.7 to 2.1 cm, and the para-aortic lymph node is essentially resolved, measuring 4 mm where it previously measured 11 mm. Two months into her complete convalescence from neurological adverse effects related to the MS flare-up, she was rechallenged with a second round of pembrolizumab therapy with a baseline of continued prednisone 20 mg, which she tolerated well without MS relapse. She subsequently encountered immune-mediated acute hepatitis and recurrent MS flare-up after cycle 4 of therapy. Labs revealed liver transaminases elevated to 20 times upper limit of normal (ULN)- AST 711 U/L, ALT 978 U/L, and ALP 392 U/L (3 x ULN) with normal total bilirubin 1.1 mg/dl. She was then subjected to another round of high-dose prednisone 1.5 mg/kg with a prolonged taper, requiring a stay at a skilled nursing facility and PT for around 1 month. Restaging PET scan after four cycles of immunotherapy in July 2019 revealed significant interval improvement: previous prominent metabolic adenopathy in the left pelvis/inguinal region and lower retroperitoneum had completely resolved. Small residual nodal lesions remained in the left inguinal region and left external iliac chain, with these demonstrating only low level/background metabolic activity. For example, a left inguinal node was 1.3 × 1.8 cm with an SUV max of 1.0 on axial image 271 - previously a nodal conglomerate in this region was 2.8 × 5.6 cm and SUV max 21.1; a small residual nodal prominence in the left external iliac region was 0.9 × 2.7 cm, SUV max 2.0 on image 256, whereas previously two adjacent nodes in this region were 3.3 × 7.6 cm and SUV max 19.1. At this point, given recurrent and severe CPI-mediated immune-related adverse effects with MS flare-ups and hepatitis, further immunotherapy was discontinued. The patient continued to exhibit an incomplete response thereafter, but with decreased quality of life given the persisting debility from MS relapse.
Malignant melanoma is a highly aggressive cancer, with only a 15-20% five-year survival rate once it has spread to the lymph nodes or has distant metastasis7. The development of ICIs has changed the face of melanoma treatment and has improved the survival rates of these patients. The drugs in this class have been directed against CTLA-4, PD-1 and PD-L1, namely ipilimumab, nivolumab and pembrolizumab, respectively3. ICIs have changed the landscape of advanced melanoma with exponential improvements in survival, the 5-year survival rates were about 50%8,9.
ICIs can present with a wide range of irAEs due to lack of selectivity and by their generalized immune reaction10. These effects range from systemic autoimmune complications to severe neurological complications11. The neurological complications have been estimated to have an incidence of 2–4% with severe neurological manifestations, including MS, being reported in 0.2–0.4% of patients treated with pembrolizumab and nivolumab11.
MS is recognized as T cell-mediated immune response causing inflammation, which causes local inflammatory plaques and demyelination8. ICIs are likely to generate an immune response that causes molecular mimicry and cross-react with CNS autoantigens, in turn exacerbating pre-existing immune response and subsequent flare-ups in MS11,12.
On an extensive literature review, we could come across very few reports of ICIs use in patients with MS. Isitan et al. described a 46-year-old with stage IV metastatic non-small cell lung cancer who was treated with one dose of atezolizumab, who subsequently developed relapse of MS with worsening features and refractory to treatment13. Another case, described by Gettings et al., was of a 56-year-old male with MS with recurrent melanoma after multiple resections; he was treated with ipilimumab who developed two MS flare-ups, leaving him with disability from MS relapse but had remission of melanoma on PET scan14. Garcia et al. conducted a retrospective analysis capturing 42,529 adverse events with the use of ICI with 13 cases identified with MS. Of the 13, eight had a previous diagnosis of MS. All the patients in their analysis experienced progression of their MS, and two patients died following their MS relapse15.
Our patient had her first MS relapse after the first dose of pembrolizumab, which resolved with high-dose steroids. She did not develop any flare-ups with the second and third rounds of therapy, but again encountered a relapse during the fourth round, which was again successfully treated with steroids but with delayed convalescence. At the end of the fourth round patient achieved complete remission of metastatic malignant melanoma. Our case adds to the pool of existing clinical data that immune checkpoint inhibitors are universally detrimental to patients with MS, given definitive relapse identified in almost all the patients identified during the literature review and our personal experience.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Multiple sclerosis, neuroimmunology
Peer review at F1000Research is author-driven. Currently no reviewers are being invited.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 23 Sep 20 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)