Keywords
Marigliano-Cacciafesta-polypathology-scale, glycaemic control, Alzheimer’s disease, delirium
Marigliano-Cacciafesta-polypathology-scale, glycaemic control, Alzheimer’s disease, delirium
This version of the article has details about the treatment with central nervous system agents in the groups of AD patients with and without diabetes. The references have been increased, considering the suggestions of the reviewers. The introduction and the discussion have been improved.
See the authors' detailed response to the review by Sandra Sigala
See the authors' detailed response to the review by Takahiko Nagamine
Behavioral and psychological symptoms of dementia (BPSD) and delirium are common in the advanced phases of Alzheimer’s disease (AD) and worsen with disease severity, whereas symptoms of cognitive decline may be prevalent in the first phases of the disease1.
Both the hyper- and hypo-glycemic extremes of the glycemic spectrum may worsen cognitive functions in AD patients2–4, and nutrition plays an important role in the development of the delirium in frail older patients5.
The aim of this preliminary report was to evaluate the relationship between delirium and glycemic control in the advanced phases of AD.
The study involved human participants with metabolic syndrome in aging and it was performed in accordance with the ethical standards of the local institutional committee (S. Andrea Hospital, ID number 67910) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent for participation and evaluation of data was obtained from the individual participants included in this study or from their caregivers, where ability to consent was affected by delirium.
We randomly (systematic sampling) enrolled 38 elderly patients (n = 11 males and n = 27 females) from the outpatients of the S. Andrea Hospital of Rome, in 2019. From their medical records, the patients met the criteria for probable AD dementia proposed by the National Institute on Aging-Alzheimer’s Association (NIA-AA)6 (Mini Mental State Examination (MMSE) score of 12.4+7.3, mean + standard deviation, in the moderate-severe stage of the disease). Prior to inclusion, subjects with other diseases, such as territorial infarction, intracranial hemorrhage, brain tumor, hydrocephalus, or severe white matter hyperintensities (WMH) were excluded from the study. Sixteen of the patients (16/38, 42% of the sample) were affected by type 2 diabetes (T2DM, according to 2020 American Diabetes Association, ADA, criteria)7, undergoing treatment with oral glucose lowering agents (n = 13, one patient with repaglinide) or with insulin (n = 3).
Each patient received from our group: a) a comprehensive geriatric assessment (CGA), including the evaluation of the Activity of Daily Living (ADL), the Instrumental Activities of Daily Living (IADL), the Cumulative Illness Rating Scale (CIRS) and the Marigliano-Cacciafesta Polypathology Scale (MCPS)8,9; b) an anthropometric evaluation, including height, weight, body mass index (BMI), waist circumference, Mini Nutritional Assessment (MNA)10; c) a behavioral and psychological examination, using the Cornell’s scale, the Clinical Dementia Rating scale (CDR), the Neuropsychiatric Inventory (NPI) and the Confusion Assessment Method (CAM, with CAM-severity)11–13. An assessment of glucose metabolism, including fasting glycaemia and insulinemia (excluded in insulin-treated patients), glycated haemoglobin (HbA1c) and insulin resistance by the means of the HOMA-index (glycemia mg/dl x insulinaemia µU/mol /405) (excluded in insulin-treated patients) and an evaluation of renal function, measuring creatinine and glomerular filtration rate (GFR) estimated using the Chronic Kidney Disease Epidemiology Collaboration formula (CKD-EPI), was performed using a clinical chemistry analyzer (c16000 Architect System, Abbott Laboratories). A continuous glucose monitoring (FreeStyle Libre system) was used in selected patients with a high risk of hypoglycemic events, or with great variability of glycaemia during the day.
For the statistical analysis, carried out using Primer of Biostatistics Version 7, one-way analysis of variance (ANOVA) was used for the evaluation of the differences between the groups of patients. The relationship between two variables was examined by the means of regression analysis. A p<0.05 was assumed as significant. Data are presented as mean + standard deviation.
The clinical characteristics of the 38 AD patients with and without T2DM are described in Table 114.
| AD patients without T2DM | AD patients with T2DM | |
|---|---|---|
| Age (years) | 83.3+5.7 | 82.3+4.2 |
| MMSE | 11.6+7.1 | 12.7+7.5 |
| ADL (IADL M and F) | 2.9+2.2 (M: 1.7+1.7; F: 1.2+1.8) | 2.7+1.8 (M: 1.4+1.5; F: 0.7+1.0) |
| MCPS | 48.6+10.5 | 47.4+7.0 |
| CIRS-SI and -CI | 2.2+0.6 and 4.8+2.2 | 2.3+0.4 and 5.6+1.4 |
| BMI | 24.4+7.0 | 26.7+5.7 |
| Waist circumference (cm) | M: 98.7+1.5; F: 90.3+22.6 | M: 97.7+8.6; F: 89.1+15.2 |
| MNA | 17.4+6.0 | 18.8+4.8 |
| Cornell | 14.2+8.6 | 11.4+6.0 |
| CDR | 2.7+1.4 | 2.2+1.1 |
| CAM | 2.7+1.5 | 2.2+1.9 |
| CAM-S | 7.8+4.1 | 4.7+3.7* |
| NPI | 53.7+28.5 | 40.2+20.2 |
| Fasting glycaemia (mg/dl) | 94.5+11.2 | 135.6+58.0** |
| Fasting insulinaemia (µu/ml) | 6.0+2.6 | 8.9+9.5 |
| HbA1c | 5.4+0.4% | 6.8+1.1%*** |
| HOMA index | 1.4+0.6 | 3.2+3.4* |
| GFR (CKD-EPI; ml/min) | 66.0+16.9 | 64.9+23.4 |
MMSE, Mini Mental State Examination; ADL, Activity of Daily Living; IADL, Instrumental Activities of Daily Living; MCPS, Marigliano-Cacciafesta Polypathology Scale; CIRS, Cumulative Illness Rating Scale; SI, severity index; BMI, body mass index, MNA, Mini Nutritional Assessment; CDR, Clinical Dementia Rating scale; CAM, Confusion Assessment Method; CAM-S, CAM-severity; NPI, Neuropsychiatric Inventory; HbA1c, glycated haemoglobin; GFR, glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration formula; M, male; F, female.
*p<0.05, **p<0.01, ***p<0.0001.
The percentage of use of drugs (antipsychotics, sleeping pills, antidepressants, antidementia, antiparkinsonian agents, mood stabilizing medications) in AD patients with or without diabetes, were respectively: 37%, 19%, 19%, 6%, 19%, 6%, and 32%, 18%, 23%, 9%, 9%, 9% (differences not significant). One AD patients with diabetes was treated with zolpidem.
The MMSE scores were inversely and significantly related to CAM-S score (r = -0.585, p<0.001), while MCPS scores were positively related to the CAM-S score (r = 0.440, p<0.05) (Figure 1a and 1b).
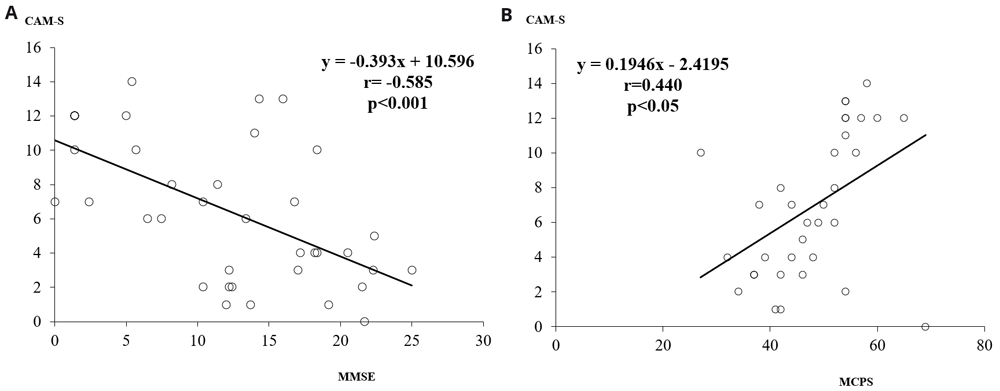
a) The relationship between MMSE and CAM-S scores. b) The relationship between MCPS and CAM-S scores. MMSE, Mini Mental State Examination; CAM-S, Confusion Assessment Method-severity; MCPS, Marigliano-Cacciafesta Polypathology Scale.
The regression analysis between HbA1c and MMSE score showed a concave downward “∩” parabola (not significant) with apex at HbA1c of 10.5% and MMSE of 13.0. The regression analysis between HbA1c and the psychometric BPSD scales (Cornell, NPI and CAM-S) showed a “U” shape (concave upward parabola) (Cornell p<0.05, NPI<0.01 and CAM-S p<0.0001) (Figure 2). The apices of the parabolas were at HbA1c of 6.9%, 7.6% and 10.3%, the levels that minimized the Cornell, NPI and CAM-S scores, respectively (Figure 2). Therefore, the mean HbA1c that minimized all the behavioural disorders was 8.3%.

Continuous glucose monitoring in selected patients (n = 3) showed that, even if HbA1c level was within the normal range, either a great variability in glycaemia during the 24 hours or repeated hypo-glycemic events during the night (up to 26 hypoglycemic events in a single patient) were present (Figure 3).

A) Patient A, 81 years, MMSE=1.5, NPI=77, Cornell=13, CAM-S=17, HbA1c=6.6%, glucose mean 150 mg/dl, above/in/under the interval 56/43/1%, event of low glucose=0, mean duration=0, mean duration=0 min. B) Patient B, 77 years, MMSE=13.7, NPI=30, Cornell=12, CAM-S=4, HbA1c=5.5%, glucose mean 72 mg/dl, above/in/under the interval 0/16/84%, event of low glucose=26, mean duration=296 min. C) Patient C, 82 years, MMSE=10.5, NPI=52, Cornell=18, CAM-S=11, HbA1c=5.7%, glucose mean 94 mg/dl, above/in/under the interval 7/54/39%, event of low glucose=4, mean duration=281 min.
In our preliminary study, polypathology and frailty was associated with the severity of delirium as demonstrated by using the MCPS scale in correlation with the CAM-S scale.
To the best of our knowledge this is the first report about the association of the MCPS and CAM-S scale, whereas the MCPS was previously evaluated by our group in patients with comorbidities and frailty9.
Glycemic control was associated with modification of BPSD and delirium in the advanced phases of AD. In particular, delirium (as indicated by the CAM-S score) was more susceptible to the glycemic control in its low range. As considered by point 7 (Metabolism and Nutritional state) of MCPS, high glucose levels increase the score of the MCPS scale (+25) only in conditions of diabetes mellitus decompensation, with significant risks of development of delirium.
Glycemic control modulated the appearance of BPSD and delirium with a polynomial U-shaped curve, with both the hyper- and hypo-glycemic extremes of the glycemic spectrum worsening BPSD. The regression equations showed that HbA1c levels between 6.9–10.3% (mean 8.3%) minimized BPSD, in agreement with International Diabetes Federation (2020)15 and ADA (2020)7 guidelines for managing T2DM in older people. As matter of fact, in healthy older subjects (with few coexisting chronic illnesses, intact cognitive and functional status) a reasonable HbA1c goal is <7.5% (with fasting or pre-prandial glucose of 90–130 mg/dl), whereas in complex/poor health patients (with more chronic illnesses, moderate-to severe cognitive impairment or >2 ADL dependencies) the reasonable HbA1c goal rises to <8.5 (with fasting or pre-prandial glucose of 100–180 mg/dl) to avoid possible overtreatment, hypoglycemic events and fall risk.
The HbA1c value of 6% corresponds to a mean glucose of 126 mg/dl, with 95% prediction limits of 100–152 mg/dl, which includes the lower goals for glycaemia (100–180 mg/dl) suggested by ADA standard of care16–18 in older frail patients with moderate-severe dementia. More than half of our patients (22/38, 58%) showed HbA1c levels lower than 6%.
The reduction of one point (%) of HbA1c (from 6.8% to 5.8%) was associated with an increase of +2.2 points in Cornell, +7.0 points in NPI and +0.2 in CAM-S scores. Because of the parabolic profiles of the curves, another reduction of one point of HbA1c (from 5.8% to 4.8%) resulted in an exponential increase of BPSD scores (+5.6 Cornell, +14.4 NPI and +2.6 CAM-S).
The variability of glycaemia seemed to be associated with greater BPSD in AD patients, although it was not possible to carry out a statistical analysis because of the few number of patients in the sample (a limit of the study).
The treatment with central nervous system drugs (antipsychotics, sleeping pills, antidepressants, antidementia, antiparkinsonian agents, mood stabilizing medications) may be a confounding factor in AD patients19–21, but, as above reported, no differences were found in the present study between the two AD groups with and without diabetes.
In conclusion, the measurement of HbA1c in elderly AD patients with advanced dementia (with and without T2DM) is recommended in order to evaluate glycemic control (and nutritional status). The reduction of mean glycemic levels (due to malnutrition in non-diabetic patients or overtreatment in diabetic patients) should be avoided by means of a multidimensional approach.
DANS EASY: The Glycaemic Control and the Severity of the Delirium. https://doi.org/10.17026/dans-xq4-58fq14.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Nagamine T: Severe hypoglycemia in an elderly patient withAlzheimer's disease and with sepsis: The role of neurotransmitters on glucose regulation. Geriatrics & Gerontology International. 2021; 21 (1): 116-117 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: psychopharmacology, emergency medicine, diabetes
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical pharmacology; translational oncology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 28 Jul 21 |
||
|
Version 1 16 Dec 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)