Keywords
oral care tablet, oral microbiome, actinidin, QIIME 2, amplicon sequence variants (ASVs)
This article is included in the Japan Institutional Gateway gateway.
oral care tablet, oral microbiome, actinidin, QIIME 2, amplicon sequence variants (ASVs)
See the authors' detailed response to the review by Priya Nimish Deo
See the authors' detailed response to the review by Rohit Kunnath Menon
See the authors' detailed response to the review by Takuichi Sato
Oral microbiota is a collection of microorganisms that reside in the oral cavity. It has been linked to the promotion of both health and diseases1,2. Among the different tissues in the oral cavity, the tongue is considered a dominant source of oral microbial populations3,4. Further, tongue coating is proposed to cause oral malodor5 or, upon sudden dissociation, aspiration pneumonia in elderly people with impaired defense mechanisms6,7. In addition, the tongue coating is a risk indicator of aspiration pneumonia in edentate individuals8.
A variety of methods to reduce tongue coating have been developed and tested to reduce oral malodor9,10. Mechanical removal of the tongue coating using tongue brushes or tongue cleaners is one such popular method9,11. Other methods include using antimicrobials, e.g., in gels or mouthwashes, or using oral tablets10,12.
The tongue microbiota in elderly individuals has been classified into several types with characteristic bacterial composition. These types correlate with the risk to aspiration pneumonia4,13. Therefore, methods that could alter the tongue microbiota to a healthy microbiota type could contribute to oral health. We have previously reported that tongue brushing does not alter the alpha or beta diversity of oral microbiota in healthy adults14,15. By contrast, according to a recent study, the use of oral care tablets decreases the amount of volatile sulfur compounds (VSCs) produced by bacteria16. Further, oral care tablets that contain actinidin, a cysteine protease found in kiwifruit, reduce oral biofilm formation in vitro12. However, it is not clear whether these interventions affect the oral microbiota as a whole or the abundance of specific bacteria.
In the current study, we examined the effect of oral care tablets with and without actinidin on the salivary and tongue microbiomes of healthy individuals. We also investigated the diversity of the salivary and tongue microbiomes, and interpersonal microbiome diversity. We show (1) that alpha diversity of the salivary microbiome was greater than that of the tongue microbiome, (2) that an individual’s salivary and tongue microbiomes were more similar to one another than to those of another individual, and (3) that the oral care tablets did not affect the oral microbiomes in the population tested. These findings add to the knowledge of the interpersonal diversity and dynamics of the oral microbiota in humans.
Ten healthy adults participated in the study, with three different treatments tested: two different types of oral tablets (with or without protease), and a negative control (no tablet). For the tablet treatments, saliva and tongue coating were collected between October 2016 and November 2017 at participants’ home (mainly in Osaka, Japan, and in some cases, nearby prefectures). DNA extraction and data analysis were conducted at the Department of Bacteriology, Osaka Dental University (Hirakata, Japan).
Participants were recruited from faculty members and graduate students working at the Osaka Dental University hospital, as well as from dentists who were acquainted with an author of this study. Ten healthy volunteers (6 males and 4 females; age: 27–60 years [39.8 ± 3.1 (mean ± SD)]) were enrolled in the study and were anonymized randomly as A–G, O, Q, and R (Table 1). The inclusion criteria were as follows: healthy men and women over 20 years of age. The exclusion criteria were as follows: daily smoking, treatment with local or systemic antibiotics within 1 month prior to the study, and allergy to kiwifruit. The exclusion criteria of one month for antibiotic treatment was set based on previous reports on the robustness and resilience of salivary microbiome17,18. For example, change in microbiome caused by exposure to clindamycin lasted up to 1 month in saliva18. According to the medical questionnaire, (1) none of the participants were undergoing or planning treatment for dental caries or periodontal disease, (2) there were no participants who were suffering from diabetes, chronic kidney disease, lung diseases, malignant tumors, etc., or who were visiting hospitals or taking medication, and (3) none of the participants experienced frequent thirst. The method and objective of this study were explained to the participants, who provided written informed consent before participating. The Osaka Dental University Medical Ethics Committee approved this study (approved on 3/31/2015; approval number 110864) and the investigations were conducted following the rules of the Declaration of Helsinki. The committee did not consider the study to be interventional in nature and therefore is not a clinical trial.
Two types of oral care tablets for tongue cleaning were tested in the current study. One type contained actinidin, a cysteine protease extracted from kiwifruit (“protease tablet”) and the other did not (“plain [placebo] tablet”). Both tablets were provided by Ezaki Glico Co., Ltd (Osaka, Japan). The protease tablets were identical to those marketed as BREO EX (Ezaki Glico Co.). Tablet composition was described previously12. To use the tablets, the participants placed one tablet on the dorsum of the tongue and waited until it dissolved naturally. One tablet takes approximately 5–7 min to completely dissolve.
The study design is illustrated in Figure 1. The tongue tablet experiment was a placebo-controlled double-blind crossover study. The 10 participants were randomly divided into 2 groups of 5 participants each, by using computer-generated random numbers. All participants performed an initial tongue cleaning (by brushing) at the beginning of the study. The participants were asked not to eat, drink, or perform oral cleaning before each sampling. After a washout period of 10 days during which the participants did not perform any tongue cleaning, they collected their saliva and tongue coating into separate containers in the morning immediately after waking up (sample D1). Then, the participants in each group took tablets, with or without the protease. The participants and the researchers who analyzed the data were not informed about the tablet types given to the participants. The participants were asked to use the tablet three times on the day of the experiment—in the morning (between 9–12 am), in the afternoon (1–4 pm), and in the evening (7–10 pm)—taking one tablet each time. The following morning, the participants collected their saliva and tongue coating separately immediately after waking up (sample D2). After a washout period of 10 days, the participants took the other type of tablet that they had not previously received, and collected the saliva and tongue samples as before. Control experiments (no tablet usage) were conducted with the same participants, after they conducted treatment using the tablets. The duration between the tablet treatments and the control experiment ranged from 10–60 days, depending on the participant. In these experiments, after an initial tongue cleaning and a 10-day washout period, all participants collected samples on two consecutive days (D1 and D2) without taking any tablet in between.
The saliva and tongue-coating samples were collected immediately after the participants woke up, in the morning of the day of tongue cleaning by tablet (D1) and the next morning (D2). The participants first collected 3 mL of saliva in a 25-mL sterile plastic tube. The tongue coating was collected by scrubbing the tongue with a swab and then soaking the tip of the swab in 0.6 mL of phosphate-buffered saline (PBS(-); Wako Pure Chemical Industries, Ltd., catalogue number 166-23555) to suspend the coating. Because this collection method involves scrubbing the tongue with a swab, the tongue coating was collected from the left half of the tongue for D1 and from the right half of the tongue for D2, to minimize the carryover effects of scrubbing. The collected samples were maintained at 4°C for up to 1 day and transported to the laboratory. The saliva samples (3 mL) were homogenized by repetitive pipetting. Then, 0.5-mL aliquots were transferred into sterile tubes. The saliva (0.5 mL) and tongue-coating (0.6 mL) samples were then centrifuged at 10,000 × g for 4 min. The supernatant was discarded and the pellet was stored at −20 °C until DNA extraction. All samples were frozen no later than on the day of D2 sampling.
Bacterial DNA was extracted from the pellets via chemical and mechanical lysis using a QIAamp UCP Pathogen Mini kit (QIAGEN, catalogue number 50214), as previously described19,20. Briefly, thawed pellets were immediately suspended in 0.5 mL of ATL buffer containing the optional DX reagent, transferred to a Pathogen Lysis Tube S, and then homogenized using a Mixer Mill MM 301 (Retsch) for 3 min at a vibrational frequency of 30 Hz. The manufacturer’s protocol was followed thereafter to complete the DNA purification. DNA was eluted in 50 μL of the AVE buffer (QIAGEN). DNA concentration was determined using a Quantus fluorometer (Promega) and a Qubit dsDNA BR Assay kit (Thermo Fisher Scientific, catalogue number Q32850). DNA was stored at –80 °C until use.
Bacterial 16S ribosomal DNA amplification and library construction were performed according to the 16S Metagenomic Sequencing Library Preparation guide supplied by Illumina (part No. 15044223_B), as previously described19. The V3–V4 region of the 16S ribosomal RNA gene was amplified by polymerase chain reaction (PCR) with a thermal cycler MJ-Mini (Bio-Rad Laboratories), using primers 341F (5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3’) and 806R (5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3’) (custom-synthesized by Invitrogen), and Premix Ex Taq Hot Start Version (Takara Bio, catalogue number RR030A). The thermal cycling conditions were initial denaturation at 98 °C for 10 s, followed by 25 cycles at 98 °C for 10 s, 55 °C for 30 s, and 72 °C for 1 min (the first PCR step). The underlined nucleotides served as primer sequence parameters to extract the V3–V4 region for feature classifier training (see next section). The amplicons were purified using AMPure XP beads (Beckman Coulter, catalogue number A63880). Sequencing adapters containing 8-bp indices were incorporated at the 3’- and 5’-ends of the purified amplicons during a second PCR step. The amplicons were again purified using the AMPure XP beads, and then quantified using a Quantus fluorometer (Promega) and a Qubit dsDNA HS Assay kit (Life Technologies, catalogue number Q32851). After pooling equimolar amounts of the amplicons, 5% of an equimolar amount of PhiX DNA (PhiX Control v3, Illumina, catalogue number FC-110-3001), was added. The obtained library was pair-end sequenced at 2 × 250 bp using a MiSeq Reagent Kit v2 (Illumina, catalogue number MS-102-2001) and the Illumina MiSeq platform. Sequencing was performed over seven independent runs at the Oral Microbiome Center (Takamatsu, Japan), followed by demultiplexing. Raw nucleotide sequences are available at DDBJ/EMBL-EBI/NCBI database under the accession number DRA010849.
Demultiplexed paired-end sequences were processed using QIIME 2 (v.2020.2) and its associated plugins21 in a Docker container. Sequences obtained from independent Miseq runs were denoised separately using DADA2 (via q2-dada2)22 applying previously-optimized parameters14 (trim-left-f = 20; trim-left-r = 20; trunc-len-f and trunc-len-r were set between 241 and 248 depending on the sequence quality; other parameters followed the default settings, including chimera-method = “consensus”). The resulting exact amplicon sequence variants (ASVs) were merged (via q2-feature-table). For taxonomy assignment to each ASV, a naïve Bayes taxonomy classifier trained (via q2-feature-classifier)23 on the V3–V4 region of the 16S rRNA sequences in the expanded human oral microbiome database (eHOMD; v.15.2)24 was used. All ASVs were aligned using MAFFT25 and used to construct a phylogeny with FastTree 2 (via q2-phylogeny)26. Sample metadata format was validated using the cloud-based tool Keemei27.
Alpha diversity was assessed by calculating the number of observed features (ASVs) and the Shannon index (via q2-diversity), after samples were subsampled without replacement (rarefied) to 40,000 sequences per sample. The non-parametric Kruskal–Wallis test was used to test for significant differences in alpha diversity between sample groups (P < 0.05).
Beta diversity was computed based on the unweighted UniFrac distance28 (via q2-diversity) and visualized as three-dimensional principal coordinate analysis (PCoA) plots using EMPeror (via q2-emperor)29. Permutational analysis of variance (PERMANOVA)30 was used to test the significant difference in bacterial composition among samples (P < 0.05). Differential abundance of bacterial taxonomic groups was tested using the analysis of composition of microbiomes (ANCOM) (via q2-composition)31.
Clustered operational taxonomic unit (OTU) table was also used to calculate the beta diversity and differential abundance of specific taxonomic groups. Open-reference clustering was chosen for a high-quality taxonomic assignment to a curated database32. Here, the entire ASVs were clustered into OTUs by open-reference picking (via q2-vsearch)32 using the V3–V4 region of 16S rRNA sequences in the eHOMD v.15.2 as a reference, with 99% identity threshold. A phylogenetic tree was constructed as described above. The R package treeio (v.1.12.0)33 was used to change the OTU names within the phylogenetic trees, as per the requirement for the Rhea package (the software disallows OTU names starting with a number). Multidimensional scaling (MDS) based on generalized UniFrac distance34 was performed to examine the difference in microbial composition among samples, using Rhea pipeline (v.1.1.3)35. For differential abundance analysis, “Serial group comparisons” in Rhea was performed (abundance_cutoff = 0.2; prevalence_cutoff = 0.3; max_median_cutoff = 1) with significance cutoff of P < 0.05 in Kruskal-Wallis test.
Box plots that show the alpha diversities were generated using R packages ggplot2 (v.3.3.2) and ggpubr (v.0.4.0), with data retrieved from QIIME 2 artifacts using qiime2R (v.0.99.31). Heatmaps and box plots that show the relative abundance of microbial taxonomies, and a Venn diagram that show core OTUs, were generated using ampvis2 (v.2.6.5)36. R (v.4.0.2) and RStudio (v.1.3.959) were used for all analyses. Software, plugins, and R packages used in this study are listed in Table 2.
| Name | Version | URL |
|---|---|---|
| QIIME 2 | 2020.2 | https://qiime2.org/ |
| Keemei | https://keemei.qiime2.org/ | |
| R | 4.0.2 | https://www.r-project.org/ |
| Rstudio | 1.3.959 | https://rstudio.com/ |
| Rhea | 1.1.3 | https://github.com/Lagkouvardos/Rhea |
| ampvis2 | 2.6.5 | https://madsalbertsen.github.io/ampvis2/index.html |
| treeio | 1.12.0 | http://bioconductor.org/packages/release/bioc/html/treeio.html |
| qiime2R | 0.99.31 | https://github.com/jbisanz/qiime2R |
| ggplot2 | 3.3.2 | https://ggplot2.tidyverse.org/ |
| ggpubr | 0.4.0 | https://github.com/kassambara/ggpubr |
The study design is illustrated in Figure 1. Ten healthy adults participated in the study, with three different treatments tested: two different types of oral tablets (with or without protease), and a negative control (no tablet). For the tablet treatments, the saliva and tongue coating were collected before (D1) and after (D2) the intervention. Overall, 116 samples were collected (10 participants, three treatments, two sites [the saliva and tongue], and two sampling time points [D1 and D2], with four samples excluded because of insufficient amount of extracted DNA). The sample metadata are provided as underlying data (Table S1)37.
DNA was extracted from each sample and the V3–V4 region of the 16S rRNA gene was PCR-amplified. The amplicons were paired-end sequenced using the Miseq platform. After quality control and error correction using DADA2, 11,260,102 reads corresponding to 5342 ASVs were obtained. Per-sample median was 89,923, with a maximum of 186,864 and a minimum of 46,422. Open-reference clustering, using the curated 16S rRNA sequences in the eHOMD v.15.2 database as the reference (at 99% identity threshold), grouped the sequences into 1210 OTUs. Either the full or clustered table was analyzed further, depending on the type of analysis performed, as described. The clustered OTU table is provided as underlying data (Table S2)38.
We first analyzed the microbiome of the saliva and tongue coating, to determine the baseline for the study. In total, 30 D1 samples (10 participants, three independent treatments) of the saliva and tongue coating were analyzed. Alpha diversity of the tongue microbiome was significantly lower than that of the salivary microbiome, using both the number of observed ASVs (254 ± 53 in saliva and 175 ± 37 in tongue; P = 8.9e-7, Kruskal–Wallis test) (Figure 2a) and Shannon index (6.0 ± 0.4 in saliva and 5.4 ± 0.3 in tongue; P = 2.0e-7, Kruskal–Wallis test) as measures (Figure 2b). This was consistent with a previous report3. The difference in diversity is probably associated with the tongue acting as a specialized niche for specific microorganisms, and the saliva containing a mixture of microbiota from different sites in the oral cavity. Interestingly, the number of observed ASVs varied among the individuals, ranging from approximately 170 to 325 in the saliva (P = 0.027, Kruskal–Wallis test), and from approximately 125 to 225 in the tongue coating (P = 0.022) (Figure 2c), suggesting a difference in oral microbiome among individual.
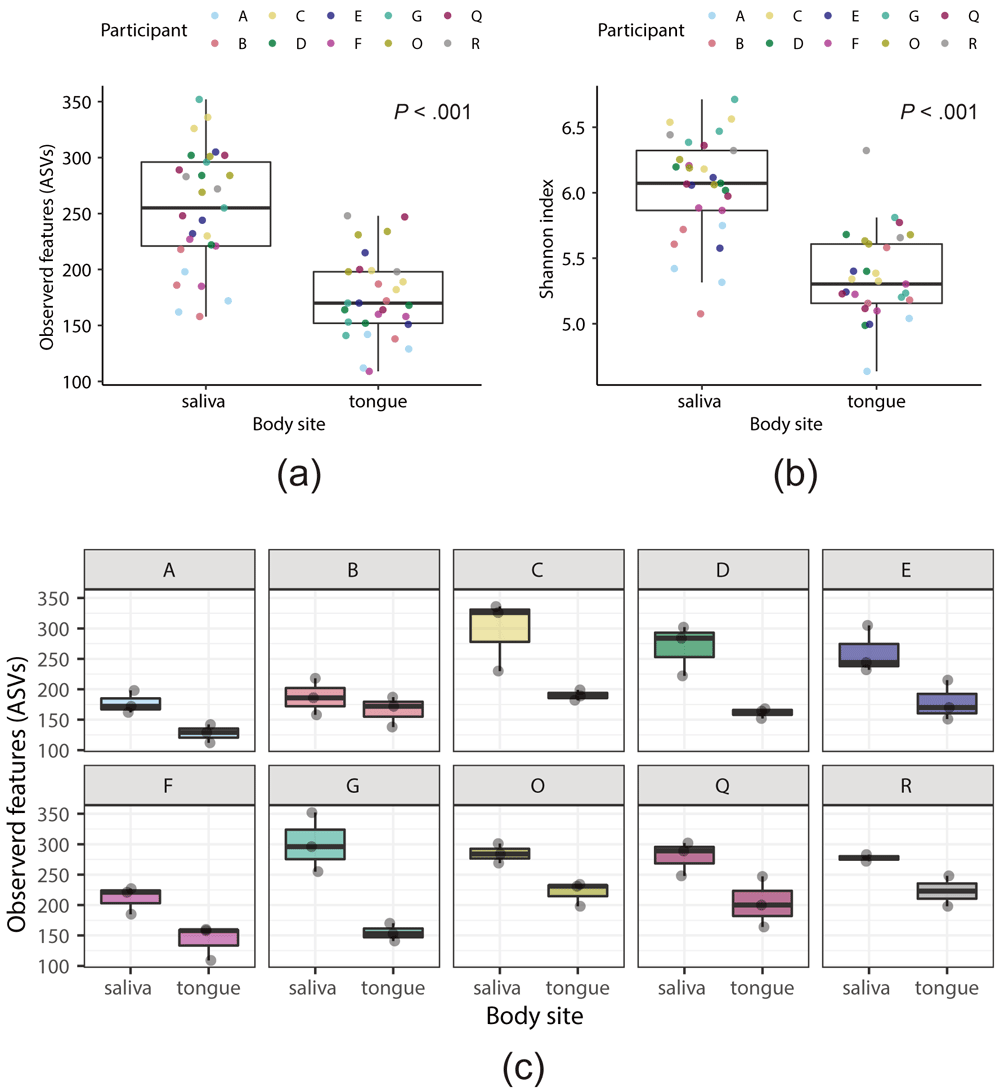
Data from the full amplicon sequence variants (ASV) table were used to calculate the alpha-diversity indexes. (a, b) Thirty samples each from the salivary or tongue microbiome were compared (three D1 samples for each of 10 participants). The number of observed ASVs (a) or Shannon index (b) was used as the alpha-diversity measure. Each point indicates a sample. Colors of the points indicate different participants. (c) Box plots of the number of observed ASVs in the salivary and tongue microbiomes (n = 3 each) for each participant.
We next assessed the differences in bacterial composition among samples (beta diversity) (Figure 3). A significant difference between the salivary and tongue microbiomes was detected both in PCoA, based on unweighted UniFrac distances using the full ASV table (P = 0.001, PERMANOVA) (Figure 3a), and MDS, based on generalized UniFrac distances34 using the clustered OTU table (P = 0.003, PERMANOVA) (Figure 3c).
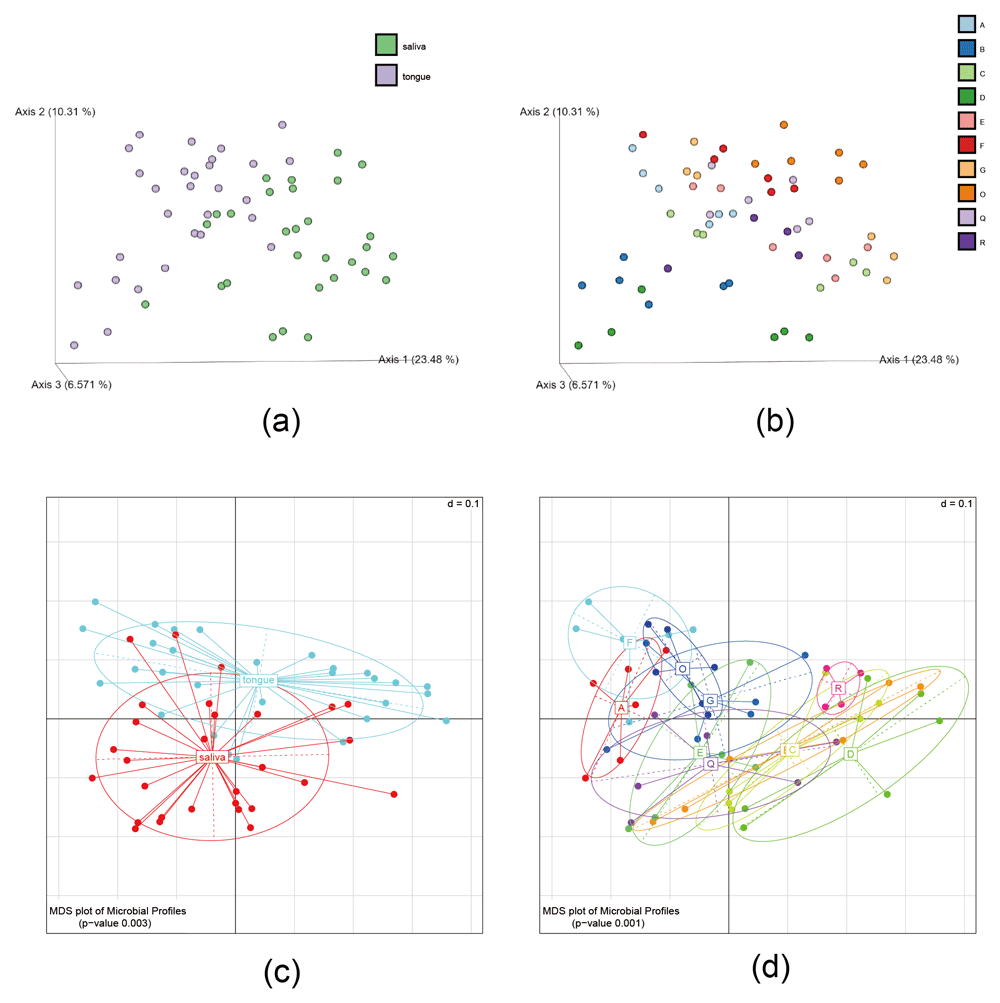
(a, b) Three-dimensional principle co-ordinate analysis (PCoA) plots were generated with EMPeror using the full amplicon sequence variants (ASV) table. While only samples corresponding to D1 are displayed (n = 58), the PCoA analysis included all (116) samples. (c, d) multidimensional screening (MDS) plot of microbial profiles calculated based on generalized UniFrac distances using the clustered operational taxonomic unit (OTU) table. Each point indicates a sample. The points are colored according to the sampling site (a, c) or the participant from whom the sample was obtained (b, d).
The data also revealed a significant difference between individual microbiomes (Figure 3b and 3d; P = 0.001, PERMANOVA using the clustered OTU table). As indicated by the plots in Figure 3, the similarity of the salivary and tongue microbiomes within an individual was greater than the similarity of the salivary or tongue microbiomes between individuals. This suggests there is stability in an individual’s oral microbiome, at least within the relatively short time period of the study (several weeks). This observation is consistent with earlier studies that highlight the stability of an individual’s oral microbiome39.
The abundances of bacterial taxonomic groups at the genus or species levels in the salivary and tongue microbiomes, determined by the analysis of D1 samples from the three treatments and based on the clustered OTU table are summarized in Figure 4a and 4b. The eight most abundant genera were common to the salivary and tongue microbiomes, accounting for nearly 80% of both microbiomes (78.2% in the saliva and 80.9% in the tongue). These genera were Prevotella (18.4% and 23.5%, respectively), Veillonella (9.0% and 12.6%, respectively), Neisseria (11.6% and 9.9%, respectively), Haemophilus (10.9% and 8.6%, respectively), Streptococcus (9.4% and 6.1%, respectively), Alloprevotella (8.2% and 5.9%, respectively), Porphyromonas (6.1% and 6.7%, respectively), and Fusobacterium (4.6% and 7.6%, respectively) (Figure 4a). The abundance of bacterial taxa at species level is shown in Figure 4b. Prevotella melaninogenica HMT-469 was most abundant in both microbiomes (8.1% in the saliva and 11.8% in the tongue), followed by Streptococcus oralis subsp. dentisani HMT-398 (7.2%) and Haemophilus parainfluenzae HMT-718 (7.0%) in the saliva, and by Fusobacterium periodonticum HMT-201 (7.4%) and H. parainfluenzae HMT-718 (7.4%) in the tongue (Figure 4b).
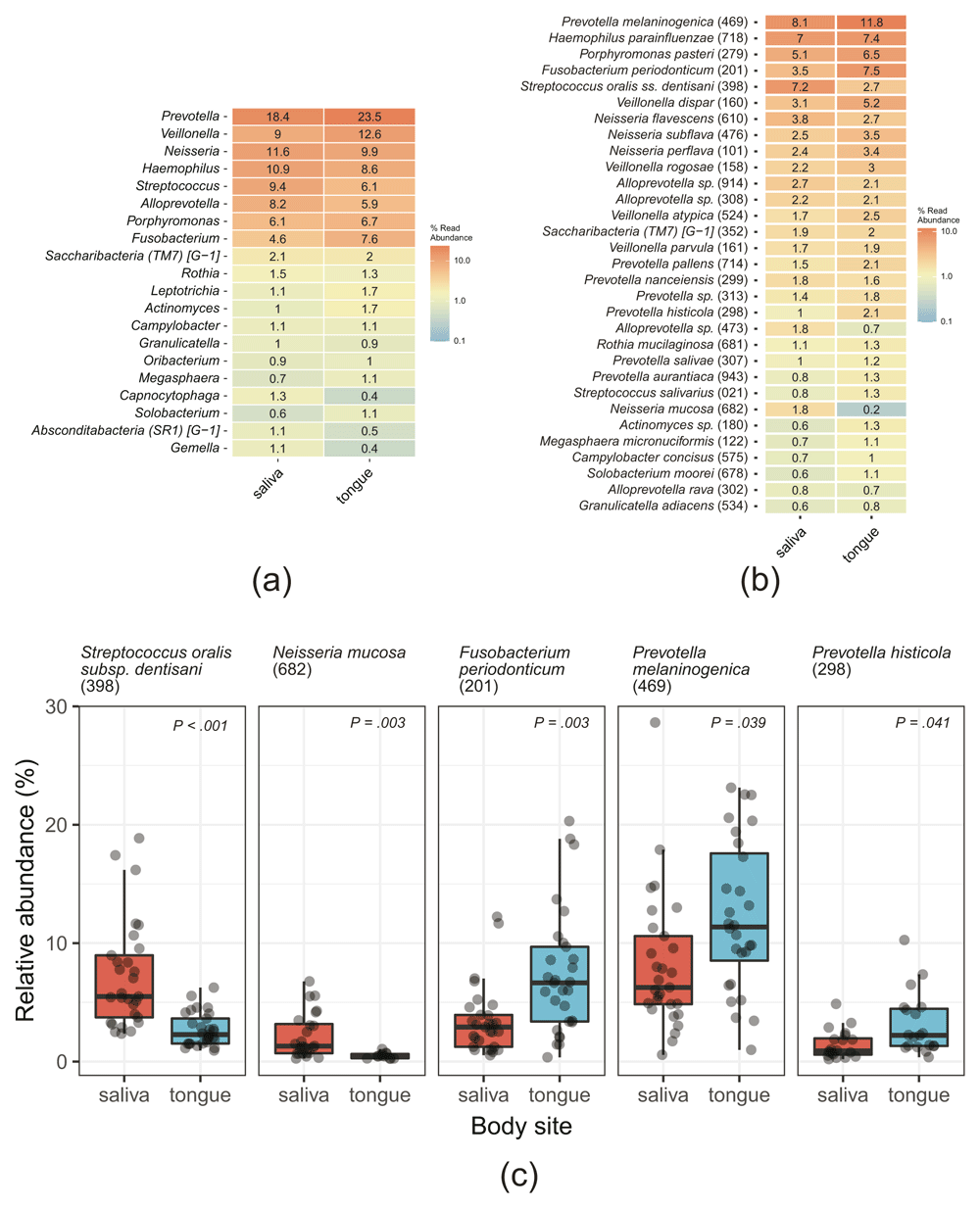
The clustered operational taxonomic unit (OTU) table was used for calculations. (a, b) Heatmaps of the abundance of bacterial taxa at the genus (a) or species (b) levels. The % read abundances are indicated, together with a color gradient. In (b), the counts of clustered OTUs with the same Taxon ID were aggregated. (c) Box plots summarizing the abundance of OTUs that were differentially abundant (P < 0.05, Kruskal–Wallis test with Benjamini–Hochberg adjustment) in the saliva and tongue samples at the species level are shown. Each dot indicates a sample. Taxon IDs in eHOMD are indicated in the parentheses.
Five OTUs were differentially abundant in the salivary and tongue microbiomes (P < 0.05, Kruskal–Wallis test) (Figure 4c). Among them, S. oralis subsp. dentisani HMT-398 (7.2% in the saliva and 2.7% in the tongue) and Neisseria mucosa HMT-682 (1.8% and 0.2%, respectively) were more abundant in the saliva, whereas F. periodonticum HMT-201 (3.5% and 7.4%, respectively), P. melaninogenica HMT-469 (8.1% and 11.8%, respectively), and Prevotella histicola HMT-298 (1% and 2.1%, respectively) were more abundant in the tongue (Figure 4b and 4c).
To identify the core members of the oral microbiome, we focused on OTUs that were present in ≥95% of the saliva or tongue D1 samples. Seven OTUs were present in ≥95% of both, the saliva and tongue samples. These were F. periodonticum HMT-201, Saccharibacteria (TM7) [G-1] sp. HMT-352, S. oralis subsp. dentisani HMT-398, P. melaninogenica HMT-469, Granulicatella adiacens HMT-534, Campylobacter concisus HMT-575, and H. parainfluenzae HMT-718 (Figure 5).
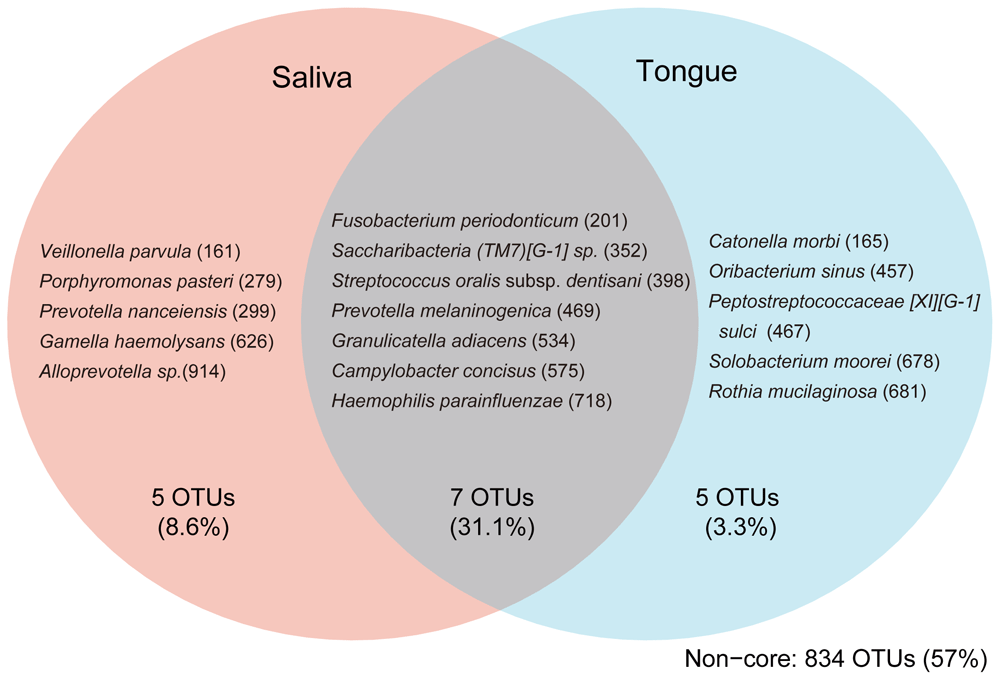
OTUs present in ≥95% of samples (D1 samples, n = 58) in the indicated subset of oral sites are shown together with the average total abundance of the OTUs in the group. For example, Veillonella parvula was found in ≥95% of saliva samples and <95% of tongue samples. Taxon IDs in eHOMD are indicated in the parentheses.
Further, we identified site-specific core OTUs, detected in ≥95% samples from one site but not from the other. The saliva-specific core OTUs were Veillonella parvula HMT-161, Porphyromonas pasteri HMT-279, Prevotella nanceiensis HMT-299, Gemella haemolysans HMT-626, and Alloprevotella sp. HMT-914. The tongue-specific core OTUs were Catonella morbi HMT-165, Oribacterium sinus HMT-457, Peptostreptococcaceae [XI][G-1] sulci HMT-467, Solobacterium moorei HMT-678, and Rothia mucilaginosa HMT-681 (Figure 5).
Using the above data as the base line, we finally assessed the effect of taking oral tablets (with or without protease) on the salivary and tongue microbiomes. Alpha diversity in D1 and D2 samples was not significantly different between any treatments (Figure 6a). In the control (no tablet) treatment, whereas the observed number of ASVs seemed to slightly increase in the saliva and to slightly decrease in the tongue, they were not statistically significant (Figure 6a). This indicates that some fluctuation of the oral microbiome may occur naturally. Further, MDS analysis indicated that the beta diversity between D1 and D2 samples was not significantly different in any treatment, for either the salivary or tongue microbiome (P > 0.7, Kruskal–Wallis test) (Figure 6B).
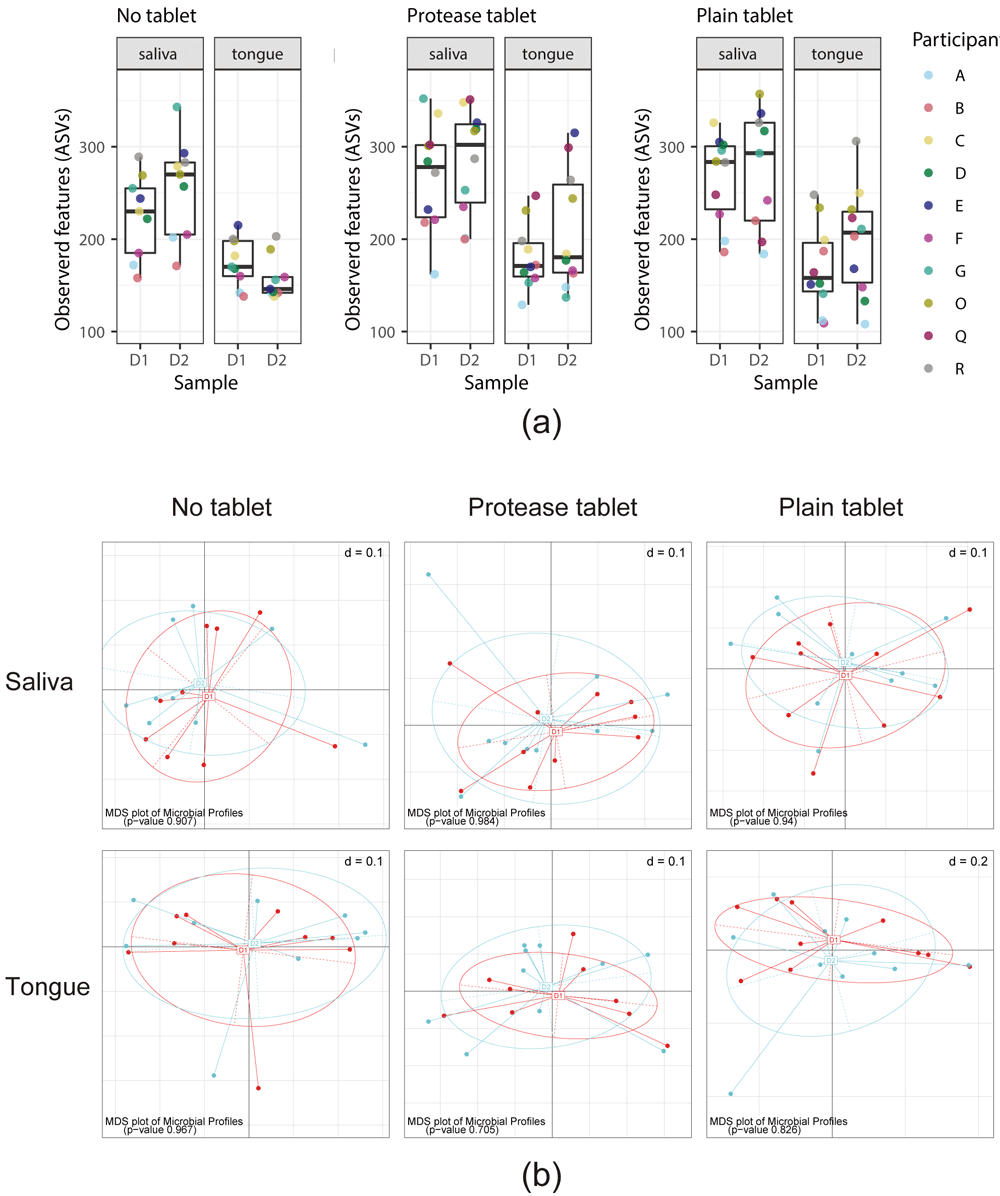
(a) Box plots of the number of observed amplicon sequence variants (ASV) in the saliva and tongue D1 and D2 samples in the no tablet (left), protease tablet (middle), and plain tablet (right) treatments. Each point indicates a sample. (b) multidimensional screening (MDS) analysis of beta diversity in D1 and D2 samples in the saliva (top panels) and tongue (bottom panels) in no tablet (left), protease tablet (middle), and plain tablet (right) treatments.
We next examined whether any bacterial species were specifically impacted by oral tablet usage. Both ANCOM using the full ASV table or Kruskal–Wallis test using the clustered OTU table revealed that no OTU was differentially abundant before (D1) or after (D2) tablet use, in any of the treatments (no tablet, protease tablet, and plain tablet). The OTU abundance in each treatment group is summarized in Figure 7a–c. Although according to a recent study oral tablet use decreases the abundance of Fusobacterium nucleatum on the tongue of healthy young adults16, we did not detect any significant decrease of OTUs that correspond to F. nucleatum. Further, in the current study, whereas 7.6% of all OTUs from the tongue microbiome were assigned to the genus Fusobacterium (Figure 4a), the majority of them were classified as F. periodonticum (7.5% of total) and only <0.1% of all OTUs was assigned to F. nucleatum at species level.
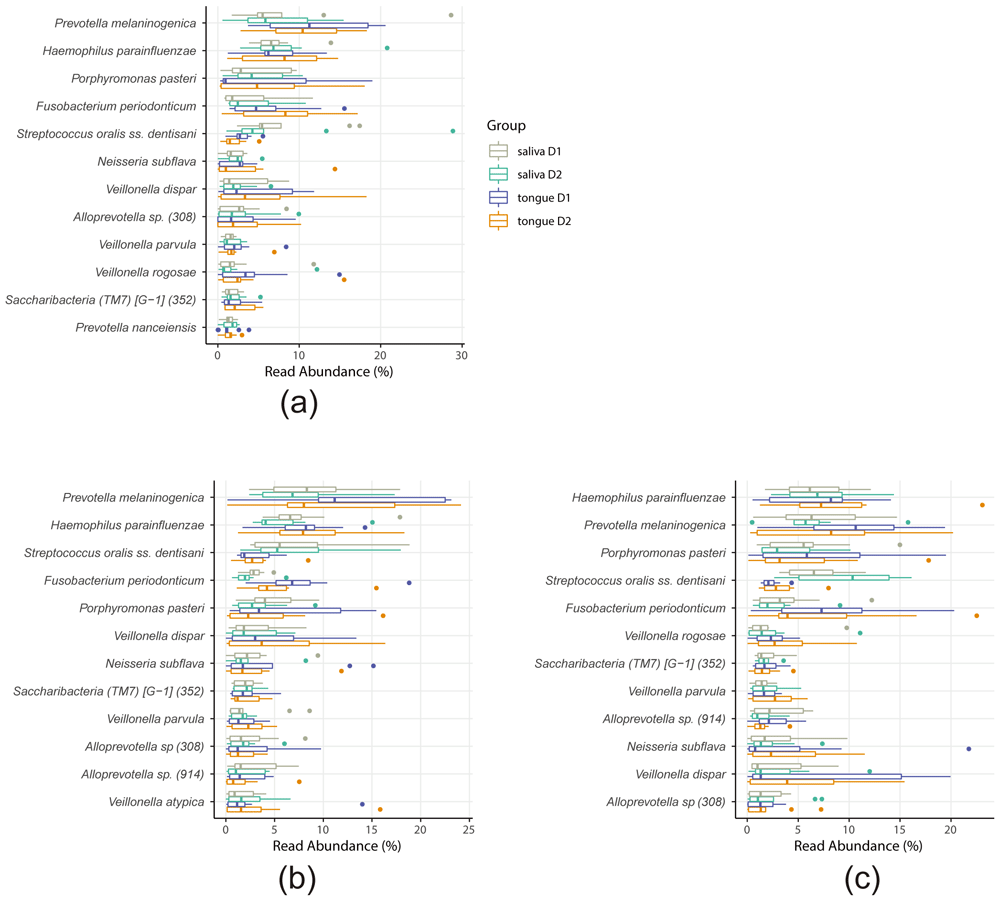
The abundances of top 12 operational taxonomic units (OTUs) based on the clustered OTU table for the no tablet (a), protease tablet (b), and plain tablet (c) treatments are shown. The data are colored depending on the group (saliva or tongue, and D1 or D2). No bacterial species showed differential abundance before (D1) or after (D2). Taxon IDs in eHOMD are indicated in the parentheses.
The oral microbiota has been associated with specific diseases in susceptible populations. In the current study, we examined the effect of oral care tablet use, with or without actinidin, on the salivary and tongue microbiomes. We showed that whereas there are some differences between the tongue and salivary microbiomes, the microbiomes were not affected by the oral tablet use, regardless of the tablet type. This does not preclude the possibility that a persistent oral tablet use would alter the oral microbiome. Controlled alteration of the oral microbiome has potential for disease prevention.
We here identify the core OTUs that are common between saliva and tongue (Figure 5). Among these OTUs, S. oralis and Campylobacter sp. have been previously determined to be the core OTUs common in the saliva and tongue3. F. periodonticum and Granulicatella adiacens have been found in tongue microbiome of adults, and P. melaninogenica and H. parainfluenzae have been found in both the infant and adult tongue40. TM7 species have been identified in oral microbiomes including tongue41,42 and supragingival plaque43. It should be noted that although the high prevalence of Saccharibacteria (TM7) [G-1] sp. HMT-352 (≥95% in both saliva and tongue), as shown in our present study, has not been reported previously, it could also be a result of clustering similar sequences into a single OTU. Although there are some differences in the classification of the core or predominant oral OTUs between the current and other studies1,3, the majority of the species were identified as the core oral OTUs across the studies. Since low-abundance rather than highly abundant OTUs may contribute more to the difference in oral bacterial communities44,45, detailed analysis of low-abundance OTUs would be important in future research. O. sinus and S. moorei were previously classified as core OTUs common to the salivary and tongue microbiomes3, but in our present study were identified as tongue-specific. This seems reasonable considering that S. moorei plays an important role in halitosis46–48. The effective separation of saliva- and tongue-specific OTUs suggest the usefulness of our study design in analyzing the salivary and tongue microbiomes simultaneously.
Our present study shows a variety among individuals in the number of observed ASVs in the salivary and tongue microbiomes. On the contrary, a significant interpersonal diversity in the supragingival plaque and salivary microbiomes, but not in the tongue plaque microbiomes was previously reported, using Faith’s phylogenetic diversity as a measure3. Since the same V3–V4 region was targeted for amplicon sequencing in both studies, the discrepancy concerning the interpersonal differences in tongue microbiome might be associated with the differences in the alpha-diversity measure used, and/or in the participants’ age (25.3 ± 3.1 years in Hall et al. study3 and 39.8 ± 10.9 years [mean ± SD] in the current study [see Methods]).
The tongue microbe is associated with various diseases, including halitosis49,50 and aspiration pneumonia4; alterations in salivary microbiome has also linked to increasing numbers of oral and non-oral diseases51. With the advancement of microbiome studies, methods to predict host traits that predispose to various diseases or conditions based on microbiome analysis have been developed52,53. Lu et al. have shown that tongue coating microbiome data can be used to distinguish individuals with pancreatic head carcinoma (PHC, one of pancreatic adenocarcinoma which occurs in the head of the pancreas) from healthy subjects41. Although the evaluation of an individual’s disease status based on the tongue microbiota data is possible, the collection methods of the tongue coating samples may not be reliable when performed by a non-specialist, especially because of the anterior to posterior gradient of the bacterial communities in the tongue surface45. We here showed that the microbiomes of the saliva and tongue of an individual tend to be more similar to one another than to the salivary or tongue microbiomes from other individuals. Considering this fact together with the stability of oral microbiome over a prolonged period of time39, salivary collection could perhaps be used in the future as a standard method to predict diseases associated with the tongue coating microbiota, as well as those linked to that of the saliva.
Oral care tablets have been previously shown to reduce tongue coating load and VSCs12,16. Here we analyzed the effect of oral care tablets on the salivary and tongue microbiomes. To avoid individual varieties in the amount of tongue coating or saliva flow affecting the analysis, we recruited only healthy adults to participate in this study. We did not detect any significant differences in the alpha diversity, beta diversity, or abundance of specific OTUs at species level after oral tablet use. There are several possible explanations for these observations. First, the participants of the current study were healthy adults with no apparent tongue coating accumulation. Although accumulated tongue coating could be reduced with oral tablets12, the amount of tongue coating analyzed herein may have been insufficient for detecting the differences in the microbiota. Second, the tablet intervention period in the current study was only 1 day and the samples were collected 1 day after the tablet use. In contrast, twice daily tongue scraping for three days, together with sampling within 15 mins after intervention, have shown to reduce the gram-negative anaerobes on the tongue9. Although we chose to collect samples 1 day after the intervention, the 1-day period could have been long enough for the resilience of the oral microbiota to revert any shift in the oral microbiomes caused by the tablet use. Considering these factors, analyzing oral care tablet intervention in individuals with a higher tongue coating index and/or over a longer period of time together with immediate sampling after intervention may provide more information on whether and how oral care tablets alter the oral microbiota, contributing to the maintenance of oral health.
The impact of external agents on microbiome depends on the location of microbiome. For example, salivary microbiome is highly resilient against external agents including antimicrobials, compared to feces microbiome that is more easily affected18. Thus, methods that can alter oral microbiome has been anticipated. Oral care tablets containing actinidin reduces tongue coating, and actinidin prevents biofilm formation by degrading cell-surface proteins in vitro12. We here attempted to elucidate the effect of the protease, supplied in oral tablets, on the oral microbiome. Unfortunately, we were unable to assess the effect of the protease, because oral tablet treatments failed to alter the oral microbiome or specific bacterial taxa, regardless of the presence or absence of actinidin. As above, including participants with a higher tongue coating and a longer intervention period with immediate sampling may have allowed detection of the effect of actinidin in the tablets. Alternatively, an in vitro culturing system could be used to analyze the effect of actinidin on the oral microbiome, with the effects of the compound tested in a controlled manner. For example, nitric oxide19 or statins54 have been shown to alter the abundance of specific bacterial species. Using such system would allow the analysis of the effect of actinidin on oral microbiota separately from the effect of mechanical removal of the tongue coating.
Various lines of evidence suggest a link between oral microbiota and health or diseases1,2,55. The current and other3 studies have highlighted interpersonal differences in the oral microbiota. Several types of tongue microbiota have been shown to exist in individuals with different susceptibility to pneumonia4. Hence, personalized treatment based on an individual’s oral microbiota is required, as has been already pointed out in the context of periodontal disease56. Analysis of how different types of oral microbiota are affected by certain interventions (e.g., oral care tablet or antibiotic treatment) would enable a more precise control over the oral microbiome in the future. In vitro culturing systems mentioned above are powerful tools for elucidating responses of bacterial communities taken from different individuals to various interventions, and the contributing factors.
In conclusion, we have shown that while the salivary and tongue microbiomes differ significantly in terms of bacterial composition, they show inter- rather than intra-individual diversity, although it should be noted that the study has a limitation in the sample size of ten individuals. We have also identified bacterial species that are common to the salivary and tongue microbiome, as well as those that are specific to either of these. In addition, we showed that oral care tablets may not alter the bacterial composition of the saliva or the tongue, at least over short periods of time in healthy individuals. Considering the link between oral microbiota and health or disease, analyzing the differences in how individual oral microbiota responds to external factors will pave the way to more effective therapeutic and diagnostic approaches and, ultimately, contribute to the development of personalized dental medicine.
Raw nucleotide sequences are available at DDBJ/EMBL-EBI/NCBI database under the accession number DRA010849.
Figshare: Table_S1_sample-metadata.tsv for "Inter-site and interpersonal diversity of salivary and tongue microbiomes, and the effect of oral care tablets". https://doi.org/10.6084/m9.figshare.13289618.v137
Table_S1_sample-metadata.tsv: Columns indicate sample ID, participant, sampling date (D1 or D2), treatment, Miseq run number, and sampling body site (saliva or tongue) of each sample. The “treatment” column indicates, no tablet (E2), protease tablet (E3), or plain tablet (E4) treatments.
Figshare: Table_S2_clustered-OTU-table.tsv for "Inter-site and interpersonal diversity of salivary and tongue microbiomes, and the effect of oral care tablets". https://doi.org/10.6084/m9.figshare.13291535.v138
Table_S2_clustered-OTU-table. After open-reference clustering, OTU table was constructed from the BIOM file using QIIME 2. Number of reads for each OTU in each sample are indicated, together with the bacterial taxonomy assigned to each OTU. OTU IDs are identical to matching HOMD Refseq IDs, except for those which did not match the database.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We would like to thank Hisako Sakamoto for preparation of DNA libraries, Makoto Taniguchi for Miseq sequencing, and Editage (www.editage.com) for English language editing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral Microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral microbiome and oral cancer
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Menon R, Gomez A, Brandt B, Leung Y, et al.: Long-term impact of oral surgery with or without amoxicillin on the oral microbiome-A prospective cohort study. Scientific Reports. 2019; 9 (1). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: oral microbiome
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral Microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 09 Apr 21 |
read | ||
|
Version 1 17 Dec 20 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)