Keywords
cornea, laser, retina, vision improvement, age-related macular degeneration
This article is included in the Eye Health gateway.
cornea, laser, retina, vision improvement, age-related macular degeneration
Age-related macular degeneration (AMD) is a leading cause of vision impairment globally1. Several FDA-approved pharmacologic treatments are available for the neovascular form of AMD (also termed “wet” AMD); in particular, anti-vascular endothelial growth factor (anti-VEGF) injections are broadly used to manage the disease by reducing its progression and, in many cases, by providing some vision improvement2. However, management of dry AMD and especially geographic atrophy (GA), late stage AMD, has proven to be more challenging. There are no FDA-approved pharmacologic treatments3 and only limited success in providing vision improvement has been obtained by use of intraocular implants4, of which only the implantable miniature telescope (IMT)5 is FDA-approved. Dry AMD procedures such as subthreshold laser therapy6 and photobiomodulation7 are directed primarily toward improving retinal function for early to intermediate stage AMD patients. Low vision aids such as prism spectacles8 and electronic glasses9 are also available but have not been broadly used by late stage dry AMD patients. The purpose of the present study is to describe a new corneal laser procedure that offers significant vision improvement in patients with late stage dry AMD.
This retrospective observational cohort study (registered with ClinicalTrials.gov NCT04349254 on 16 April 2020) was completed in conformance with ethical principles of the World Medical Association Declaration of Helsinki. The study protocol (Pro00044329) was approved on 4 August 2020 by an institutional review board (Advarra, Aurora, Ontario, Canada). Written Informed consent, with a provision for release of medical records, was obtained from each patient prior to treatment. Patients were primarily referred by optometrists and treated in the clinic during the period February through November 2018. Clinic records of 32 eyes of 17 patients [8F, 9M; mean (± SD) age: 81.7 (± 8.8) y; range: 58–96 y; all of Caucasian ancestry] with late stage dry age-related macular degeneration who had received one treatment in each eye using the same device and protocol and with 12-month (12m) follow-up examinations were identified and patient records were examined during the period August through October 2020. Eyes were either pseudophakic or phakic with no visually significant cataract. All patient eyes had vision impairment, with mean ± SD best spectacle-corrected distance and near visual acuities (BCDVA and BCNVA) of 20/238 (1.08 ± 0.37 logMAR; 31 letters) and 20/199 (1.00 ± 0.29 logMAR; 35 letters), respectively. The study size was limited by the availability of records but the size was confirmed to provide statistical significance of outcome measures.
Inclusion criteria included age of ≥ 55 y and, in the eye to be treated, diagnosed late stage dry AMD, moderate to profound BCDVA impairment (in the range of 20/44 to 20/1000), normal corneal topography (i.e., without distorted or unclear mires) and examinations extending to at least 12 months post-Tx. Exclusion criteria included previous corneal surgery and visually significant ocular disease other than AMD.
Examinations included slit-lamp biomicroscopy; optical coherence tomography; subjective manifest refraction (SMR); BCDVA and BCNVA; potential visual acuity (PVA)10 using Gonzalez-Markowitz charts (Precision Vision, Woodstock, IL) at 50 cm examination distance. For a subset of eyes (n=12), additional examinations included: 1) contrast sensitivity (CS) using Pelli-Robson charts (Precision Vision, Woodstock, IL), 2) corneal topography (CT) and ray tracing aberrometry (RTA) using an iTrace analyzer (Tracey Technologies, Houston, TX) and 3) retinal sensitivity, fixation stability and preferred retinal locus using a Macular Integrity Assessment (MAIA) microperimeter (MP; Centervue, Fremont, CA). SMR, BCDVA and BCNVA examinations were completed pre-Tx for 32 eyes and at 1 month (1m), 3m, 6m, and 12m post-Tx for 29, 28, 28 and 32 eyes, respectively; SMR measurements were recorded for all eyes pre-Tx but only for 12 eyes post-Tx. PVA examinations were completed pre-Tx for 20 eyes. Other examinations (CS, CT, RTA and MP) were completed pre-Tx and at post-Tx times extending to 12m post-Tx for 12 eyes (CS, CT and RTA) and 8 eyes (MP).
Treatments were completed using a Clear-K® Low Vision Aid System (Optimal Acuity Corporation, Austin, TX) to deliver pulsed laser energy simultaneously to the cornea in 4 spots of 0.5 mm diameter arranged symmetrically 90° apart and located on a 6.0 mm diameter ring centered on the pupillary centroid as shown in Figure 1. Laser parameters included 2 µm wavelength, 150 ms pulse duration and 48 to 50 mJ energy per spot. Laser light was transmitted from the console through an optical fiber array terminated by a handpiece that docks onto a sapphire applanation window/suction ring (SAWSR) assembly mounted on the eye. Laser energy was delivered through the SAWSR onto the eye in order to provide a fixed location of treatment spots with epithelial protection (by the sapphire window acting as a heat sink) from thermal damage. Treatments produced small corneal changes in shape that acted to redirect light onto functional regions of the retina. Patients were reclined to a supine position, given a drop of topical anaesthetic in the eye to be treated, and then treated.
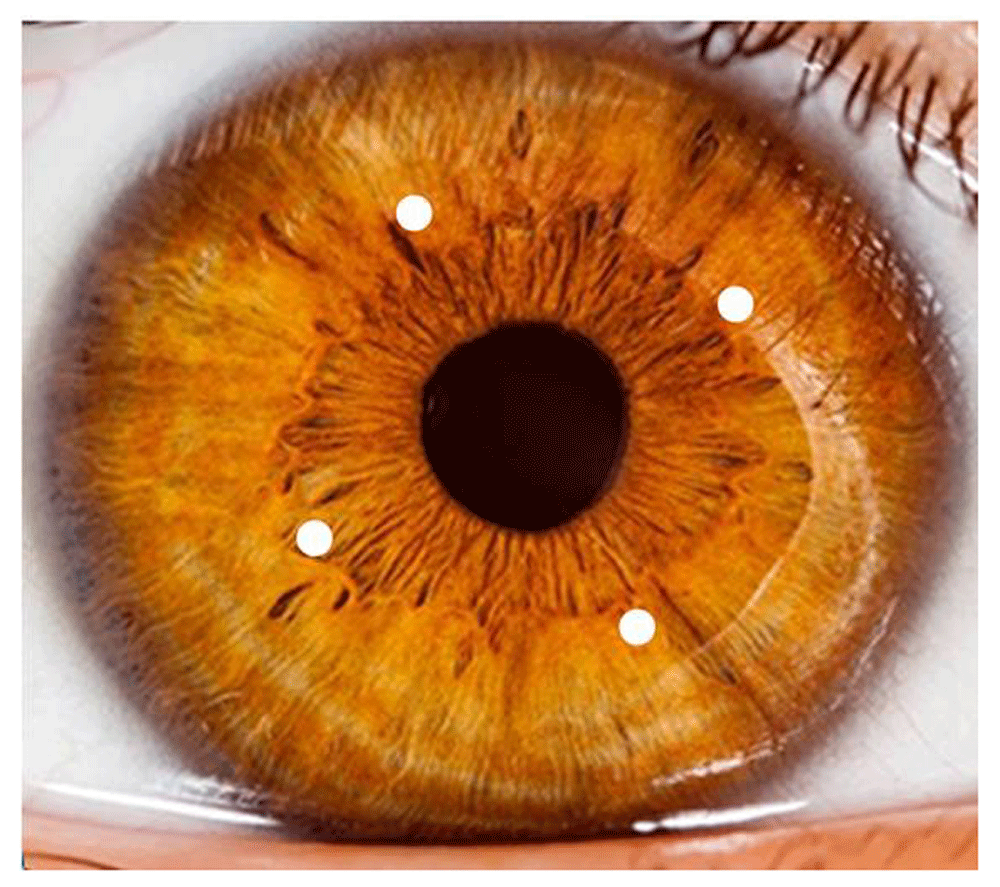
Actual Tx spots are barely visible under room lighting conditions.
Statistical significances of paired outcomes were assessed by Wilcoxon signed rank tests. Intereye correlations in bilateral treatments and correlations between potential visual acuity test measurements and visual acuity changes were assessed by Pearson correlation coefficients; statistical significances of the correlations were evaluated by bootstrap resampling. OD and OS logMAR values for correlated bilateral treatments were averaged for each outcome (BCDVA and BCNVA at baseline and at each follow-up time) in order to calculate statistical significances of post- vs. pre-Tx differences11. Many outcomes are reported as mean (± standard deviation) values. Microsoft Excel software (2010) functions were used for statistical analysis.
The mean BCDVA and BCNVA of the treated eyes increased from baseline at each follow-up (f/u) time, as shown in Figure 212 in terms of mean letters of vision gained on standard eye charts and in Figure 312 in terms of a histogram of percentages of changes in lines of vision at 12m post-Tx. Table 112 summarizes descriptive statistics of outcomes for bilateral treatments [calculated with intereye correlation, since Pearson correlation coefficients are positive and large (mean: 0.6) for all outcomes], for unilateral treatments, and for all (unilateral plus uncorrelated bilateral) treatments. For all treatments (and for bilateral Txs only), all outcomes are statistically significant at the p<0.05 level. The largest mean (± SD) gain of 14.6 (± 11.1) letters in BCDVA was achieved at 1m post-Tx; the mean BCDVA gain was stable at ca. 11 to 12 letters from 3m to 12m post-Tx. 58.6% (17 of 29) of treated eyes gained 15 or more letters (3 or more lines) of BCDVA at 1m post-Tx compared to baseline; this success percentage decreased to 43.8% (14 of 32 eyes) at 12m post-Tx, possibly due to regression of treatment effect and/or progressive dry AMD vision loss. Figure 212 also shows the expected mean BCDVA loss for untreated late stage AMD eyes for a similar cohort from another study13, amounting to 4.1 letters lost at 12m. So, the 11.9 letter mean gain in BCDVA at 12m post-Tx for CPV treated eyes is actually 15 letters mean better vision than expected for untreated eyes.
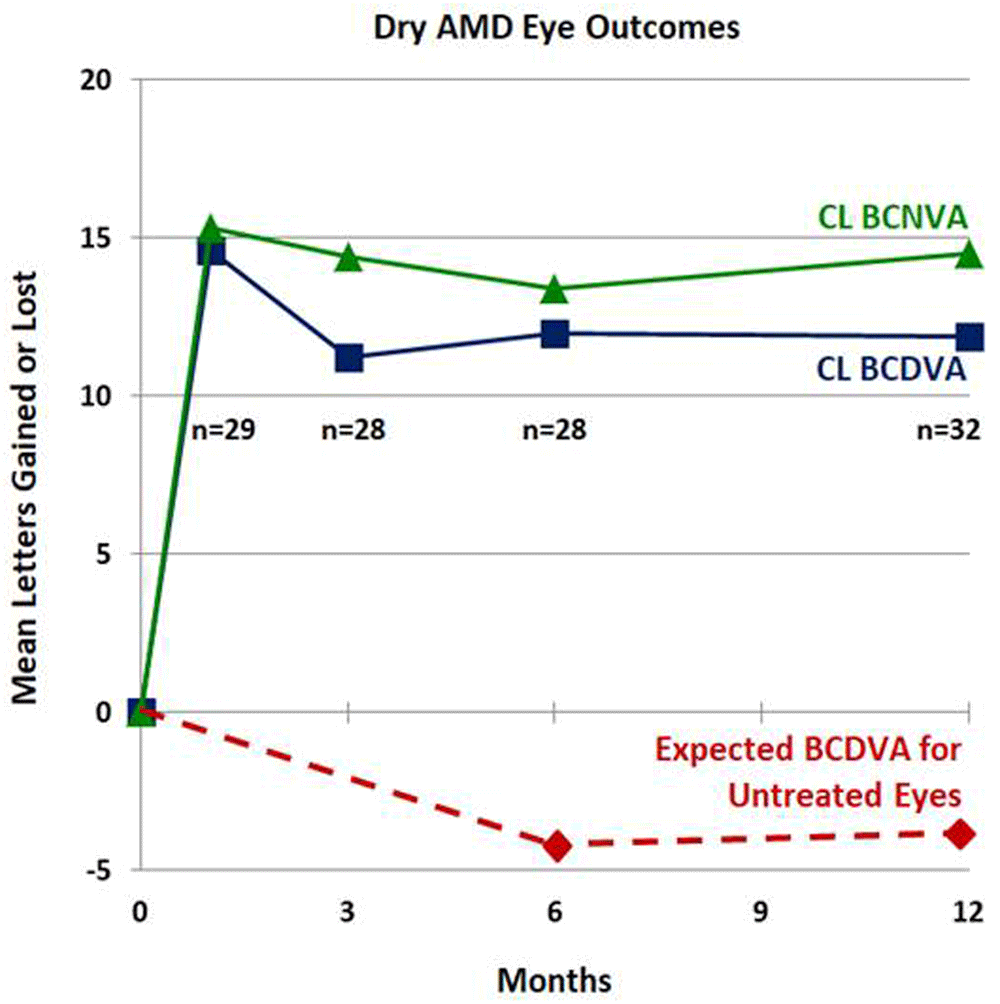
Bottom timeline – Mean BCDVA letters lost for a similar cohort of untreated eyes (from Ref. 13).
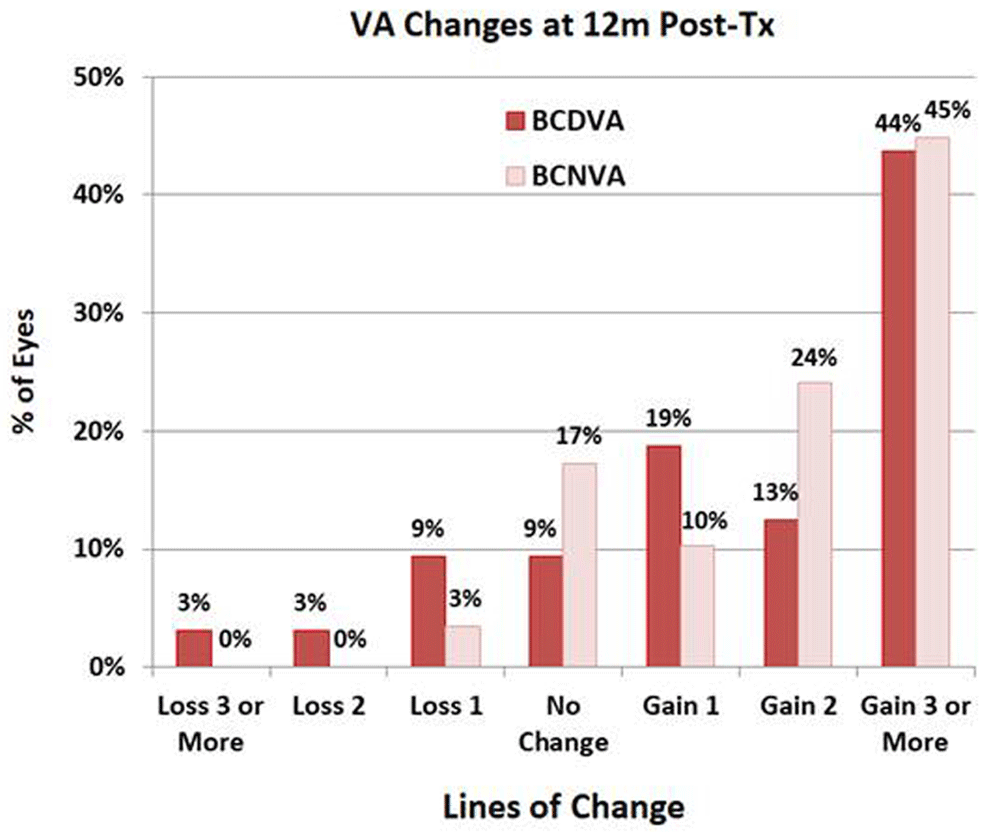
The histogram shows the percentage of eyes that lost 1, 2 or 3 or more lines of vision, were unchanged, or gained 1, 2, or 3 or more lines of best corrected distance visual acuity (BCDVA) and best corrected near visual acuity (BCNVA).
Entries contain the mean and standard deviation of letters gained, sample size (n) and p-value (where appropriate).
Pre-Tx potential visual acuity (PVA) measurements10 demonstrated variable improvements compared to pre-Tx BCDVA measurements: 6 eyes had less than 10 letters (2 lines) improvement, 6 eyes had between 10 to 14 letters (2 to 2.8 lines) improvement and 8 eyes had 15 or more letters (3 or more lines) improvement. 1m post-Tx mean BCDVA improvements correlated moderately well (Pearson correlation coefficient = 0.44; p < 0.03) with pre-Tx mean PVA improvements. The Pearson correlation coefficient = 0.32 (p < 0.12) for 3m post-Tx mean BCDVA improvements with pre-Tx mean PVA improvements was also moderately good. Pearson correlation coefficients for 6m and 12m post-Tx mean BCDVAs compared to pre-Tx mean PVAs were near-zero and were not statistically significant. The decrease in correlation may be caused by partial loss of treatment effect and, in part, by the progressive loss of BCDVA in dry AMD eyes. In the “best” mean PVA improvement group (with 15 or more letters improvement relative to baseline BCDVA), the mean (± SD) post-Tx BCDVA improvements were 21.0 (± 12.1) and 15.2 (± 13.6) letters at 1m and 3m, respectively, compared to lesser BCDVA improvements of 8.8 (± 12.0) and 5.4 (± 8.7) letters at 1m and 3m, respectively, for the PVA improvement group of 14 or less letters improvement relative to baseline BCDVA.
The mean binocular BCDVA and BCNVA values also increased significantly compared to baseline values. No symptoms of aniseikonia, polyplopia or dysphotopsia were found in all or most patients although one patient had aniseikonia that was probably due to a large difference between spectacle lenses. Amsler grid tests typically demonstrated line straightening and reduction and/or relocation of dark and missing areas.
Slit-lamp biomicroscope examination of treated corneas showed that treated spots were indented and lightly opacified; corneal epithelia were intact. Goldmann applanation tonometry measurements showed that mean intraocular pressures were unchanged at each post-Tx time.
Mean monocular contrast sensitivity (CS; at ca. 1 cycle/degree) of the treated eyes, as measured under photopic conditions without glare, increased as a function of follow-up time [significantly (p < 0.05) at all f/u times] – e.g., from log CS (mean ± SD) = 0.73 ± 0.40 pre-Tx to 1.00 ± 0.33 at 12m post-Tx, representing a mean CS increase by a factor of 1.86 from baseline. Mean binocular CS also increased as a function of f/u time by amounts similar to monocular increases.
Pre-Tx, the mean subjective manifest refraction (SMR) of the subset (n=8) of treated eyes with both SMR and aberrometry measurements was -0.41 D sphere - 0.81 D cylinder X 113°, 2.50 D add; -0.81 (± 0.46) D spherical equivalent (SE). At 1m post-Tx, the mean SMR was -0.31 D sphere - 0.53 D cylinder X 90°, 2.50 D add; -0.58 (± 0.43) D SE corresponding to a mean (± SD) hyperopic SE shift of +0.23 (± 0.33) D from baseline. The changes in SMR values from pre-Tx to 1m post-Tx were small, as were SMR changes from baseline to 3m and longer post-Tx. At 12m post-Tx, the mean (± SD) SMR SE change from baseline was -0.98 (± 0.76) D. None of the SE changes were statistically significant.
Ray tracing aberrometry (RTA) measurements provided information on retinal irradiance distribution modifications (IDMs) and objective refraction (OR) changes. Pre-Tx, rays of light incident on the cornea produced a “tight” pattern of retinal irradiation. Post-Tx, rays of light incident on the 3 mm optical zone (OZ) of the cornea were redistributed outward from the pre-Tx pattern center on the retina by a maximum mean (± SD) IDM value of 37 ± 20 µm. Pre-Tx, the mean OR of the treated eyes with aberrometry measurements was 0.16 D sphere - 1.65 D cylinder X 85°; -0.67 (±0.93) D SE. At 1m post-Tx, the mean OR was -0.22 D sphere - 1.92 D cylinder X 101°; -1.18 (± 0.77) D SE corresponding to a mean (± SD) myopic SE shift of -0.51 (± 0.52) D from baseline by aberrometry (in contrast to the SMR mean hyperopic SE shift of 0.23 D). At 12m post-Tx, the mean (± SD) aberrometry myopic SE shift from baseline was -0.12 (± 0.86) D, a smaller shift than the SMR myopic SE shift. Mean (± SD) total aberrations increased from 0.42 (± 0.21) µm at baseline to 0.53 (±0.33) µm and 0.44 (± 0.27) µm at 1m and 12m post-Tx, respectively. Most of the aberrometric changes were due to increased lower order (defocus and astigmatism) changes; both defocus and astigmatism increased. None of the total aberration changes were statistically significant.
Corneal topography (CT) measurements provided information on corneal refractive changes, averaged within the 3 mm optical zone to calculate an Effective Refractive Power (Eff RP) and Astigmatism (Astig). Pre-Tx, mean (± SD) Eff RP and Astig values were 44.22 (± 1.09) D and 1.51 (± 1.21) D, respectively. At 1m and 12m post-Tx, mean (± SD) Eff RP changes were 0.39 (± 0.32) D and 0.22 (± 0.45) D, respectively, while mean (± SD) Astig changes were 0.04 (± 0.67) D and 0.06 (± 0.45) D. None of the Eff RP or Astig changes are statistically significant.
Detailed (non-averaged) CT changes were obtained from CT difference maps. Figure 412 shows sample CT difference (1d post-Tx minus pre-Tx) maps in terms of Z Elevation and Refraction changes. In the left panel of Figure 412, depressions are evident in the Z Elevation map with maximum depressions centered on treatment spots that are located on the 6 mm optical zone (OZ). Maximum depressions vary from spot to spot in the range of -10 to -22 µm, with a mean (± SD) of -15.5 (± 5.2) µm. In the right panel of Figure 412, refractive increases are evident in the Refractive map with maximum increases on ca. the 4 mm OZ. Maximum refraction increases vary from spot to spot in the range of 1.0 to 2.1 D, with a mean (± SD) of 1.44 (± 0.51) D. At 1m post-Tx, Z Elevation and maximum Refraction changes decreased to mean (± SD) values of -10.6 (± 3.7) µm and 0.90 (± 0.38) D, respectively. At 12m post-Tx, the Z Elevation and maximum Refraction changes decreased further to mean (± SD) values of -7.4 (± 4.1) µm and 0.71 (± 0.46) D, respectively. For all eyes with CT measurements, the mean Z Elevation changes from baseline were approximately -18 µm at 1d post-Tx, decreasing to -12 µm and -8 µm at 1m and 12m post-Tx, respectively. The corresponding mean Refraction changes from baseline were approximately 2.1D, 1.1 D and 0.7 D at 1d, 1m and 12m post-Tx, respectively.
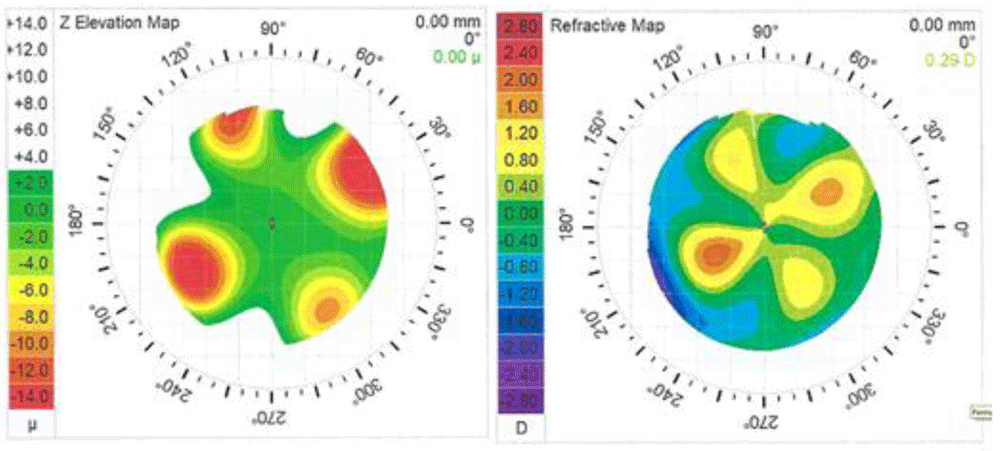
Grids display 1 mm increments. Left map: Tx spots are located at 6.0 mm optical zone (OZ). Right map: maximum refractive changes are located at ca. 4 mm OZ. Tx spots were located as shown in Figure 1.
Microperimetry exams showed that fixation stability (FS) improved post-Tx, as determined by 95% bivariate contour ellipse area (BCEA) measurements; the 95% BCEA is the area containing 95% of the fixation points during a microperimeter scan of ca. 6 minutes duration. The mean (± SD) 95% BCEA decreased from 68.6°² (± 57.9°²) pre-Tx to 42.7°² (± 30.3°²) at 1m post-Tx and 31.5°² (± 25.1°²) at 4m post-Tx, with near statistical significance (p = 0.09 and 0.05 at 1m and 4m, respectively). The Pearson correlation coefficient between 95% BCEA and BCDVA (in logMAR units) was large (0.63) for all 30 FS data points. Four of the eight eyes with MP exams had large shifts (5.3° ± 1.9°) in preferred retinal loci (PRLs) that typically reduced the overlap of fixation points with atrophied regions of the retina. (Two of the eyes did not have usable 1m exams but did have usable 3m exams that showed large PRL shifts.) Figure 512 shows an example of the PRL change in one eye due to CPV Tx. Two of the eight eyes had almost no post-Tx shifts in PRLs but these were eyes with good pre-Tx FS and almost no post-Tx FS change.
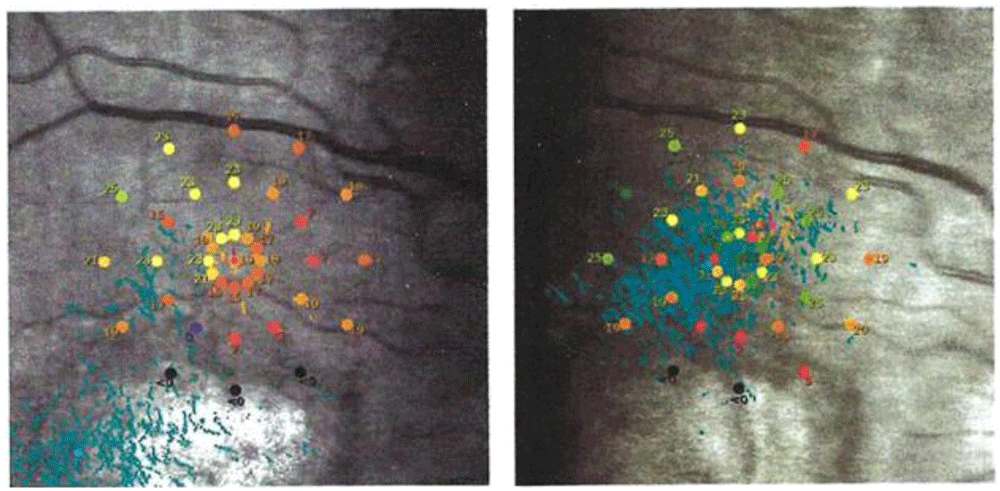
Pre-Tx: the distribution of fixation points (blue dots) was centered at bottom left with most of the points within the atrophic retina (in white). 3m Post-Tx: the distribution of fixation points was centered superiorly with respect to the atrophic retina. Retinal sensitivity measurements (shown in color coding at 37 stimulus points over 10° diameter) – the black (zero sensitivity) stimulus points overlap the atrophic retina. The distribution of fixation points moved from overlap with the atrophic area (pre-Tx) superiorly onto a more functional retina (3m post-Tx). The distribution of fixation points also decreased from a 95% Bivariate Contour Ellipse Area (BCEA containing 95% of the fixation points) of 171.6°² pre-Tx to 46.2°² at 3m post-Tx.
Laser irradiation of the cornea by CPV Tx is a new corneal laser procedure that differs from invasive corneal procedures such as conductive keratoplasty (CK)14, laser-assisted in situ keratomileusis (LASIK)15, laser thermal keratoplasty (LTK)16 and small incision lenticule extraction (SMILE)17 in many ways including that, for CPV Tx, no corneal tissue is punctured, cut or removed and the epithelium is protected from thermal damage. The laser is “eye safe” meaning that the laser light is completely absorbed in the cornea; none of the laser light propagates through the cornea to irradiate the lens or the retina. The regions of corneal refraction change shown in Figure 4 act as aspheric multifocal lenses to redistribute light entering the cornea in order to produce a retinal irradiance distribution modification (IDM) for vision improvement. Laser Raman spectromicroscopy measurements18 indicated that tissue in CPV treated spots has reduced water content, probably leading to tissue compaction. Atomic force microscopy measurements18 indicated that tissue in CPV treated spots has increased modulus, leading to a change of treated anterior corneal stroma from a gel-like state with viscoelastic character to a more glass-like state with elastic character. Tx spots are lightly opacified and are confined to the anterior stroma upon slit-lamp biomicroscopy examination; Tx spots are not cosmetically significant under normal room lighting. Since Z Elevation and Refraction changes persist, although decreasing as a function of follow-up time, it is likely that CPV effects of reduction of water content and increase of modulus also persist. None of the eyes in this pilot study have received more than one CPV Tx so the possible benefit of multiple CPV Txs on eyes (treated simultaneously and/or sequentially) to maintain or even increase vision improvement remains to be investigated.
The corneal shape and refraction changes described above produce prompt redistribution of visible light similar to “prismatic effects” produced by prism spectacles8. Unlike prism spectacles, however, the four treatment spots create a quatrefoil pattern of corneal shape and refractive change (Figure 4) for 360 degrees that resembles four lenses, which each redistributes light rays. Pre-treatment, a location in visual space that maps onto an area of dysfunctional retina in an AMD patient is perceived as part of a blind spot. However, after CPV treatment, light rays from this location are mapped onto four different regions of the retina. If any of these regions are functional, then visual information about that location is transmitted to the central brain. The success of a “one size fits all” procedure for a patient population with different geometries of dysfunctional regions on their retinas results from the relatively high probability that at least one of the four lenses will enable the retina to transmit information about any location in visual space with compromised vision.
Ray tracing measurements indicate that the displacement of images is ca. 40 µm. This is not a large enough shift to move images from the fovea all the way to functional areas of the retina in most of our patients. However, this shift can allow images near the boundary of dysfunctional areas to move onto functional areas, and thereby restore some visual function. In addition, CPV treatment produces refractive changes over a large portion of the central cornea (cf. Figure 4). These large refractive changes distribute blurry versions of images in blind spots over a larger region of the retina, thus reaching more functional areas of the retina. In addition, ray tracing measurements were made for on-axis light beams; off-axis light beams are likely to experience a larger shift in their location on the retina.
It is notable that the eye movement strategy of patients changed. Six of the eight patients with microperimeter (MP) exams experienced large shifts in their preferred retinal locus (PRL) of fixations. Typically, the new PRL was displaced away from atrophied areas of the retina, and thus allowed the patient to gain more central visual information with each saccade. (An example of this pattern is shown for one eye in Figure 5). It is unclear how the changes in retinal irradiance distribution produced by CPV Tx helped eyes to find a better PRL. One possibility is that before treatment, the motor learning system of patients was in a “false optimum”, in which small deviations from the fixation location resulted in decreased visual function. Then, following treatment, visual information was spread over a larger region of the retina, helping the motor learning system to find a “true optimum”. Further study is needed to determine patterns (and causes) of PRL changes following CPV treatment.
Another significant change in eye movements following treatment was an improvement in fixation stability, which may make it easier for the brain to perform spatial integration of visual information. These changes may also be related to a shift in the PRL, as patients making saccades into a damaged area of the retina may partially compensate by making larger fixational eye movements to attempt to scan images over functional regions of the retina. Therefore, the change in eye movements can be thought of as helping to restore more normal visual function. [It should be noted that the fixation stability observed in patient eyes with AMD in this study is much worse than in normal eyes (mean 95% BCEA = ca. 3°² for patients in their 80s19), so the treated eyes are still in need of further functional improvement.] Further study is needed to determine if and how CPV treatment improves fixation stability.
A potential concern is that the quatrefoil pattern of refractive changes produced by CPV Tx could result in multiple or fragmented visual perception. However, patients experienced unified vision. The visual information transmitted via multiple lenses combines, as each lens transmits the same information about a single location in visual space onto multiple regions of the retina. Functional combination of this information is akin to spatial integration, which the brain routinely carries out and which can result in acuity far greater than the spacing between cone photoreceptors20,21. This combination can also be thought of as a form of vision multiplexing22, which is an example of the wider phenomenon of sensory cue combination23–25. While the mechanism of optimal sensory cue combination remains an area of active research, the phenomenon is well-established.
Since potential visual acuity (PVA) measurements10 correlate well with near-term (1m post-Tx) BCDVA improvements, PVA screening may be very useful as predicted previously26. Patient expectations may also be guided by PVA measurements.
There were no safety problems associated with the minimalist corneal laser procedure used in this study. CPV Tx produces minimal corneal changes without risks of intraocular surgery.
The only FDA-approved device for vision improvement in dry AMD patients is the implantable miniature telescope (IMT)5. At 12m post-implantation, IMT patients achieved mean improvements of 3.47 and 3.18 ETDRS lines for BCDVA and BCNVA, respectively, from baseline5, mostly because of telescopic magnification inherent in the procedure. Since all the IMT patients had cataracts, the IMT study authors attributed ca. 1 line of BCDVA improvement to cataract removal27, so the net BCDVA vision improvement in IMT patients was ca. 2.5 lines at 12m post-implantation. Patients with CPV Txs achieved mean improvements of 2.2 and 2.7 lines of BCDVA and BCNVA, respectively, at 12m post-Tx without the risks and safety problems associated with the very invasive IMT surgery and without added magnification. It should also be noted that the IMT produces “tunnel vision” in the implanted eye, while CPV treatment does not.
For CPV Tx, the “best” mean PVA improvement group (with 15 or more letters PVA improvement relative to baseline BCDVA) achieved a mean post-Tx BCDVA improvement of 21.0 letters at 1m post-Tx. The large standard deviations associated with each group and follow-up time (cf. Table 1) may be due, at least in part, to the range of accuracy with which light rays are redirected onto functional retinal areas. In this pilot study, each eye received the same CPV Tx. The possible benefit of custom CPV Txs (for example, by changing the pattern and energy density of Tx spots) that increase the accuracy of light ray redistribution onto the most functional retinal areas in each eye remains to be investigated.
CPV treated patients typically experienced rapid and comfortable Txs with no post-Tx requirements for new medications or visual rehabilitation training (as is the case, for example, for IMT patients5). The present CPV study involved both unilateral and bilateral Txs, depending on whether one or both eyes needed vision improvement. A CPV bilateral Tx regimen contrasts with the IMT unilateral Tx regimen that is required because the IMT device produces “tunnel vision” and patients need an untreated fellow eye for peripheral vision and ambulation5.
Another CPV study28 demonstrated that bilateral Txs of both wet and dry AMD eyes produced similar vision improvements as in the present study. There is considerable merit to using combination therapy for wet AMD eyes in which anti-VEGF injections reduce the progression of the disease and CPV Tx provides significant vision and vision-related quality of life improvements.
Limitations of the present pilot study are:
1 – small sample size,
2 – retrospective analysis of outcomes and
3 – follow-up of only 12 months post-Tx.
A prospective clinical study on a larger patient cohort with inclusion of additional measurements over a period extending to 24 months post-Tx is planned.
The CPV procedure for vision improvement is a new modality that may be broadly useful to benefit patients affected by late stage AMD.
Vision improvement outcomes plus additional measurements
DRYAD: Data from: Corneal laser procedure for vision improvement in patients with late stage dry age-related macular degeneration. https://doi.org/10.5061/dryad.sn02v6x2x12
This project contains the following underlying data:
- F1000Research_Dataset_1_-_Corneal_laser_procedure_-_with_age_ranges.xls (Vision improvement outcomes (BCDVA, BCNVA, PVA and CS) and additional measurements (SMR, CT, RTA and MP))
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors gratefully acknowledge Robert Devenyi, MD, FRCSC, Ghani Salim, MD and Chiara Cinco for fine discussions, documentation help and supplementary information.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: ophthalmology, corneal surgery
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: laser surgery of the eye
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 23 Dec 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)