Keywords
hypercholesterolemia, cinnamon, high-fat feed, quail yolk, body weight of mice
This article is included in the Agriculture, Food and Nutrition gateway.
hypercholesterolemia, cinnamon, high-fat feed, quail yolk, body weight of mice
In version 2 of the abstract manuscript, we revised in background by added more information about the needed to treat hyperlipidemia to prevented metabolic and CV diseases, and the importance of found natural remedies that might potentially be antihyperlipidemia agents with minimal side effects. The aims of study had confirmed.
The added information in methods about the used of male mice as research subjects to avoided bias caused by hormonal differences between male and female mice.The used of quail yolk as High Fat (HFC) food to increase cholesterol levels in mice. The quail yolk consumed was based on information from literature and preliminary studies. Details of the results with the addition of the mean value ± SD in the cholesterol level of each groups, but detailed information was not disclosed in the mice's body weight results because the results did not significantly difference. We have compiled new words to conclude this research for the better as part of the conclusion. We stated that the study was conducted for 28 days but we gave Cinnamomum Burmani for 14 days.
See the authors' detailed response to the review by Tahereh Farkhondeh
Hypercholesterolemia is a state where the cholesterol level in the body exceeds the normal range. Hypercholesterolemia can increase the risk of atherosclerosis, coronary artery disease, pancreatitis, diabetes mellitus, liver disease and renal disease, etc1.
Data from the Indonesian Ministry of Health in 2019 reported that coronary artery disease to be the first killer, 26.4% of all deaths in Indonesia2. Out of the 17 million the premature death (under the age of 70) due to non communicable disease, most cases are in low-and middle income countries, and 37% are caused coronary heart disease and 6.7 million of these were triggered by stroke (WHO, 2015)3.
Many side effects caused by the condition of hypercholesterolemia need good care besides improving lifestyle by reducing the intake of high fat foods, also taking drugs, such as statin drugs. Ezad et al. (2018) reported that the use of statin drugs can cause side effects such as myopathy, increase levels of liver enzymes, increase the risk of impairment or memory loss, and can even be fatal caused rhabdomyolysis. Therefore, it is important to find natural remedies that are efficacious in reducing cholesterol levels with minimal side effects4. In Indonesia, the development of traditional medicine is performed by exploring herbs known to be beneficial for health, and this information is passed from generation to generation. One such herb is gambier (Uncaria gambir Roxb.)5, which has antioxidant effects and lowers blood glucose when treating type 2 diabetes mellitus (T2DM). There are also bangun-bangun leaves (Coleus amboinicus), which are believed to be effective in relieving pain when distilled as ethanol and water extract6. Traditional medicine that comes from plants is commonly used by many people in Indonesia. One of these herbs is cinnamon (Cinnamomum burmannii). It has the efficacy to lower blood glucose levels. It is potential as an antidiabetic because it contains high levels of cinnamaldehyde. Cinnamon is often eaten in daily food. Cinnamon is a native plant in Indonesia and can be found abundant in Central of Java, (Karanganyar), West Sumatra (Padang), Jambi (Kerinci), etc7.
The consuming high fat feed can increase cholesterol in the blood. Lipid levels of yolks of quail are higher than chicken egg yolks8. Preliminary studies have shown success in creating hypercholesterolemia mice models by consuming high-fat feed using quail yolk. Mice were used in this study, because they have biology similar to humans, and therefore can be a model for human hyperlipidemia9–11. This study was conducted to determine the efficacy of Cinnamon burmannii in lowering total cholesterol levels of mice (Mus musculus) given high-fat feed.
This is an experimental study in an animal model with a pre-post control study design. This study was conducted at the Pharmacology Laboratory of the Faculty of Medicine, Universitas Sumatera Utara, Indonesia.
This study was approved by the Health Research Ethical Committee of Universitas Sumatera Utara (No:55/TGL/KEPK FK USU-RSUP HAM/2019).
Mice were given high-fat containing food (HFC) to create hyperlipidemic mouse model. The intervention was carried out by giving different CE doses to find the dose that reduces the total cholesterol level of the mice. Cholesterol total levels were obtained from the blood by cutting the mice’s tail. To ensure the experimental animals remained in a comfortable condition, the mice were given anesthesia procedures before cutting off the tail12,13.
The animals were purchased from the Department Biology of Mathematics and Scientific Faculty USU. In total, 30 male white mice (Mus musculus), Swiss Webster strain, 10 to 12 weeks old, and weighing 25 – 40 g were used. Before conducting the study, the mice were adapted to their cages (plastic (30 × 20 × 10 cm) and covered with fine wire mesh; base of the cages was covered with rice husks as thick as 0.5 – 1 cm and replaced every day during the study) for 2 weeks before the experiment started. They had 12 hours of daylight (6:00 A.M. – 6:00 P.M.) and 12 hours of dark (6:00 P.M. – 6:00 A.M.). The mice were fed with standard feed (CP 551) from PT Charoen Pokphand-Indonesia and water was given ad libitum. Room temperature and humidity were kept at normal ranges. They were weighed once a week to avoid stress.
After the adaptation period, the mice were randomly divided into five groups, with each group consisting of 6 mice.
The sample size was calculated according to Federer’s formula14:
(t-1) (n-1)>15
t = the number of groups
n=the number of samples
All 30 mice were given a number (1–30) using a SPIDOL marker pen, then randomized by putting the numbers in an envelope and dividing them into 5 groups according to the numbers are taken from the envelope9.
Groups were as follows: 1) K0, negative control group/placebo, no treatment or high-fat food (only given aquadest 0.5 ml); 2) K1, positive control group, diet of HFC (quail yolk); 3) K2, HFC + cinnamon extract (CE) dose 2mg/20g body weight (BW); 4) K3, HFC + CE dose 4mg/20gBW; 5) K4, HFC + CE dose 8mg/20gBW.
The study was conducted for 28 days.
Cinnamon extract. CE was given to the mice every morning at 8:00 am. Animals were kept during research in the mice laboratory in the Pharmacology laboratory. Before being given CE, it was dissolved with aquadest. The extract was given to the mice orally using feeding tubes. CE interventions began on the 15th day until the 28th day.
Cinnamon extract (Cinnamomum burmannii) was obtained as Herbilogy Cinnamon Extract Powder® (PT.Phytochemindo Reksa, Bogor, Indonesia; batch no. 033CT).
High-fat containing food. The quail yolk was used to induce hypercholesterolemia, because the concentration of the lipid in quail yolk is higher than chicken egg yolk8. A dosage of 0.5ml/day was given until day 28. The administration of quail yolk was done orally using feeding tubes as per the administration of CE.
Determination of dose of cinnamon. Based on research by Vanessa et al. in 2013, who saw a decrease in total blood cholesterol levels in white rats (Rattus norvegicus) by administering instant cinnamon powder drink (Cinnamomum burmannii BI.) at a dose of 14.4 mg and 43.2 mg for 7 days. In their research, it was found that a dose of 14.4 mg can reduce cholesterol levels in rats. Therefore we used a dose of 14.4 mg, because it was effective and efficient15. We converted its dose to mice and increased it with variation doses of 2, 4 and 8 mg. The value converted from 200g rat to 20g mice is 0.14.
Measurement of total cholesterol and BW of mice was performed on days 0, 14 and 28 (every two weeks). Anesthesia ketamine/xylazine 0.1 ml was injected intraperitoneal before the mice’s tail cutting off12,13.
Mice tails were cut 2 – 5mm to draw blood, which was then assessed for total cholesterol using the digital autocheck® cholesterol measurement tool. Mice were weighed using a digital scale.
Data were analyzed using SPSS 24. The number average of sample data presented as mean ± SD. The one-way ANOVA statistical analysis to indicate the effects of treatments for all group. If the result is significant (p < 0.05), then bootstrapping was performed using post-hoc Bonferroni for differentiating between each group.
Figure 1 shows that groups K0 (negative controls), K1 (positive controls), K2 (CE 2 mg/20gBW), K3 (CE 4 mg/20gBW), and K4 (CE 8 mg/20gBW), had a decrease in total cholesterol levels. There is a significant difference in total cholesterol among the groups (p=0.001 between groups). A post-hoc Bonferroni test was performed to see the difference in total cholesterol averages within each group.
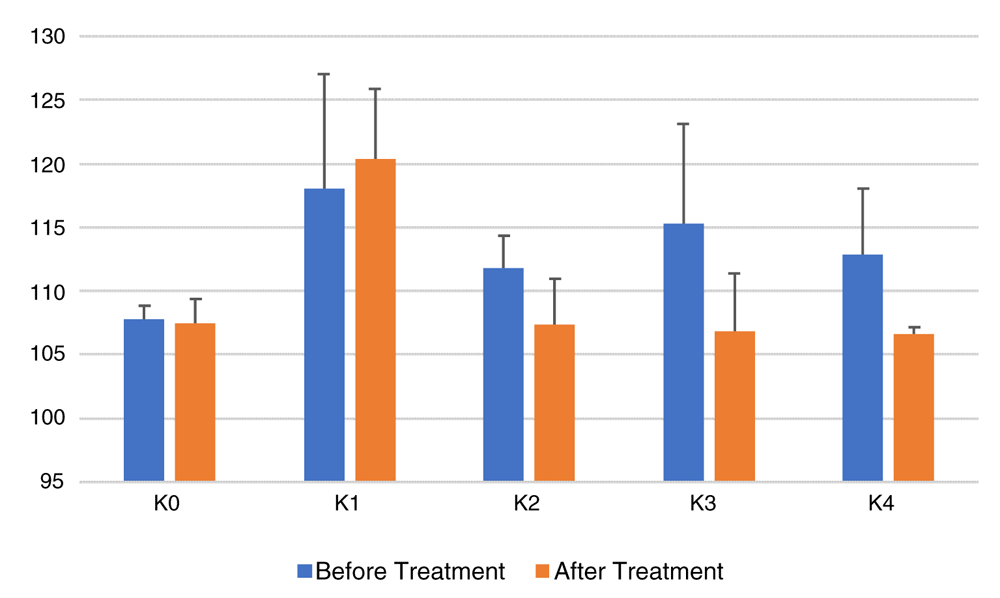
K0, negative control group, no treatment or high-fat food (aquadest); K1, positive control group, diet of high-fat containing food (HFC; quail yolk); K2, HFC + cinnamon extract (CE) dose 2mg/20g body weight (BW); K3, HFC + CE dose 4mg/20gBW; K4, HFC + CE dose 8mg/20gBW.
It was seen that there was a difference in average total cholesterol levels between K0 (107.5 ± 1.87 mg/dl) vs K1 (120.3 ± 5.53 mg/dl), (p = 0.001). This shows that in the K1 group (positive control) given quail yolk succeeded in increasing cholesterol in mice with a significant difference compared to the K0 group (negative control), which was only given aquadest. Besides, differences in total cholesterol levels in the K1 group (120.3 ± 5.53 mg/dl) compared with K2 (107.3 ± 3.61 mg/dl), K3 (106.8 ± 4.57 mg/dl) and K4 (106.7 ± 0.51 mg/dl) groups showed that a significant decrease in total cholesterol levels (p = 0.001). This proves that CE is efficacious in reducing total cholesterol levels, while in groups K2, K3, and K4, which were all given CE, there was not a significant difference between total cholesterol levels (p > 0.05).
Figure 2 shows that there were increases in BW in all groups. The largest increase of BW was in group K2, which was the positive control group who were given HFC quail yolk at 0.5 ml/20gBW. The smallest increase in BW was found in group K4, which was the group provided with HFC quail yolk and CE with the dose of 8 mg/20gBW (highest dose in this study). One-way ANOVA showed that there was no significant difference in BW between groups (p = 0.419), which could be inferred that the action of giving CE gave no effect increasing BW in mice.
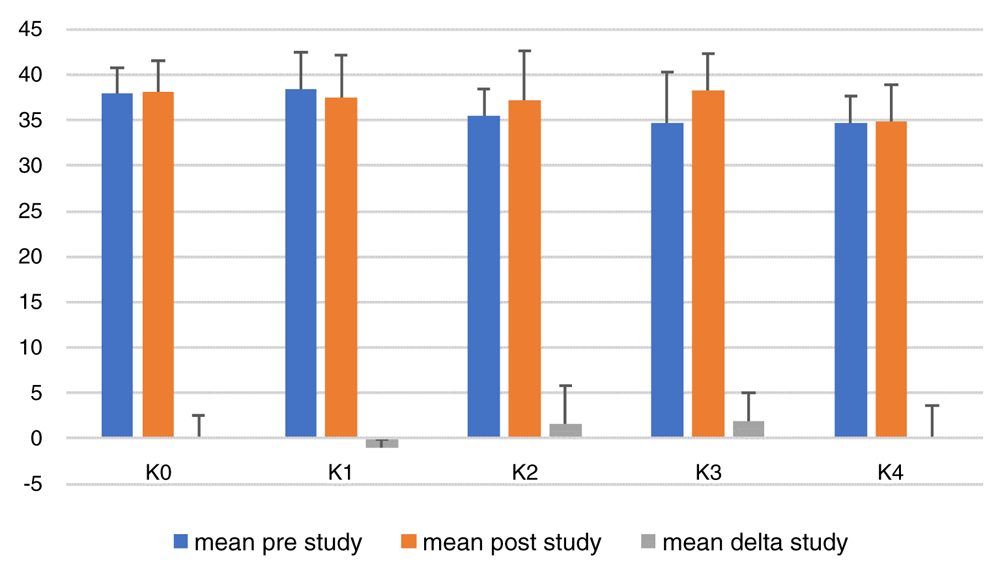
K0, negative control group, no treatment or high-fat food (aquadest); K1, positive control group, diet of high-fat containing food (HFC; quail yolk); K2, HFC + cinnamon extract (CE) dose 2mg/20g body weight (BW); K3, HFC + CE dose 4mg/20gBW; K4, HFC + CE dose 8mg/20gBW. *Mean delta = (average BW at the end of the study) - (average BW at the beginning of the study).
The aim of study to investigate cholesterol levels can be lowering in mice given variation of doses CE after consumption of quail yolk for a high-fat diet. Mice was used in this study because they have a similar biology to human, and can therefore be a model for human hyperlipidemia9–11. In this study, it was proven that consumption of 0.5ml quail yolk for 28 days increased the total cholesterol level between the negative and positive control groups (post-hoc Bonferroni K0 vs K1; p=0.001).
There was a significant difference in the total decrease in cholesterol after treatment among all groups in this research. However, this is not consistent with Vanessa et al. (2013) who stated that there was a decrease, eventhough the difference is not statistically significant. Their studies were only done for 14 days, with an increase of 3x the initial dose (14.4 mg to 43.2 mg), while our study was carried out 28 days, leading to differing results15.
Cholesterol is formed by the action of HMG-CoA reductase enzyme (3-hydroxy-3-methylglutaryl-CoA). If hypercholesterolemia is left without implementing proper diet or treatment, it can cause occlusion in a blood vessel. Hypercholesterolemia treated to medicines, such as statin could be given16. The use of statins will competitively block HMG-CoA reductase and efficiently reduce serum LDL cholesterol. But, treatment using statin can cause rhabdomyolysis4. Thus, it needed research on traditional or herbal medicine, e.g. CE, that needs developed because CE is predicted to have minimal adverse effects.
Cinnamon (Cinnamomum burmannii) has cinnamaldehyde as it’s biggest compound. Cinnamaldehyde, a phenolic component abundantly found in Cinnamomum7. Bandara et al. (2011) stated that cinnamon had the ability to be an antioxidant, antivirus, antifungal, antimicrobe, antitumor, and can lower cholesterol and blood pressure17. Cinnamon is believed to have a direct role in lipid metabolism to prevent hypercholesterolemia and hypertriglyceridemia, as well as in preventing free fatty acids with its strong lipolytic activity18. In the present study we believe that the increase of cholesterol levels due to quail yolk could be decreased with cinnamon maybe caused by its ability to block HMG-CoA reductase enzyme and suppress lipid peroxidation through increased antioxidant enzyme activity19. Abeysekera et al. (2017) reported cinnamaldehyde that highest compound in bark extracts of Ceylon cinnamon possess moderate cholesterol esterase and cholesterol micellization inhibition and bile acid binding in vitro. It could lower cholesterol levels because it contributed to bile acid synthesis20.
Our study showed that there was no difference in BW of the mice between groups (p = 0.419). This is not consistent with Vafa et al. (2012) stated that consuming 3 grams of cinnamon for 8 weeks could decrease some biochemical and anthropometric variables compared to previous states significantly, i.e. a decrease of 1.19% body weight, 1.54% body mass index and 1.36% body fat. They conducted clinical study in T2DM patients who were given cinnamon supplements (Cinnamomum zeylanicum). In addition to lowering BW variables, it could also decrease fasting blood glucose level by about 9.2%, and 6.12% of HbA1c and 15.38% of triglyceride levels21. In contrary, Alsoodeeri et al. (2020) reported weight gain in rats (Albino rats) which are given Cinnamomum cassia after consuming high fat compared to body weight at the beginning until end of the study in each groups18.
Leaf and Antonio (2017) stated that the higher the food intake (with Fat Mass/Fat Free Mass), the higher the increase in BW22. In the present study, in addition to standard food intake, giving hypercholesterol and interventional food should increase BW, because the mice had increased energy intake. The amount of food intake will affect the amount of energy intake, which will be saved as fat and impact on the mice’s BWs, since energy intake is inversely proportional to physical activity. A high-fat diet group has low sensitivity to leptin, which results in increased appetite and food intake, thus increases the BW16. However, this was not the case in our study.
In the present study showed cinnamon (Cinnamomum burmannii) extracts proved can be lowering of total cholesterol level for 14 days after consuming high-fat containing foods in mice models compared to does not given cinnamon.
Figshare: data Analysis-cholesterol and bodyweight of mice.docx, https://doi.org/10.6084/m9.figshare.11901174.v223
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The author gives appreciation and thanks to the Pharmacology Laboratory of the Faculty of Medicine, Universitas Sumatera Utara for allowing the author to use the facility for collecting data in this research. In addition, thanks to all lecturers and examiners who had given advice and suggestions to the author, and all other parties who had contributed to this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: metabolic disorder
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Vafa M, Mohammadi F, Shidfar F, Sormaghi MS, et al.: Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients.Int J Prev Med. 2012; 3 (8): 531-6 PubMed AbstractCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Ethnopharmacology, medicinal plants, diabetes, hypertension
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: metabolic disorder
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 29 May 20 |
read | |
|
Version 1 06 Mar 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)