Keywords
Randomised controlled trial, Trial methodology, Systematic reviews, Schizophrenia, Multiple sclerosis.
This article is included in the Research on Research, Policy & Culture gateway.
Randomised controlled trial, Trial methodology, Systematic reviews, Schizophrenia, Multiple sclerosis.
1. We have changed the title to 'Learning from Cochrane systematic reviews: what improvements do these suggest for the design of trials?'
2. The addition of two sections of text to the Conclusions section: 'Previous research found that small, underpowered studies make up the entirety of evidence in most meta-analyses reported by Cochrane reviews7. Only 35% of the reviews in our sample mentioned sample size in the Implication for research, which suggests that reviewers may be underestimating the size of this problem. The most frequent issue raised in our work was the choice of outcome, a persistent problem that led to the COMET initiative to facilitate the development of core outcome sets5,8. Cochrane reviewers need to continue to highlight trial design problems; indeed they could perhaps do so more often, especially around sample sizes.'
'There are resources that can help with the above. For example, COMET (http://www.comet-initiative.org) can help with outcome choice and PRECIS-2 can help with design decisions around comparators and follow-up9. Discussions with staff at clinical trial units and other research support centres are also likely to improve designs. Change is slow at present and initiatives to encourage, or force, trialists to consider these questions would be welcome, especially from funders.'
3. The addition of three new references (Refs 7-9) to support the above.
4. Provided two additional files containing all the data we extracted. These are available at https://doi.org/10.17605/OSF.IO/P9CW6
See the authors' detailed response to the review by Livia Puljak
See the authors' detailed response to the review by Christian Gluud
Randomised trials are at the heart of evidence-informed healthcare systems. Not all trials, however, are created equal. Some are excellent, others have serious methodological flaws that fatally undermine their results and make the research wasteful1–3. This is of particular importance for the systematic reviews that should be used to underpin decision making in health care, and systematic reviewers often identify relevant issues and suggest ways to improve the quality of future trials. Cochrane is an international organisation that aims to provide high-quality information to support health decisions by systematically reviewing research, especially from randomised trials to investigate the effects of healthcare interventions. It is organized across more than 50 Cochrane Review Groups, each of which looks after a particular area of health.
Cochrane pays great attention to the methodological quality of both the reviews and the studies they include4. Cochrane systematic reviews all have a section called ‘Implications for research’, which allows the authors of the review to present their conclusions on how future research might be improved, for example by discussing the types of interventions that need more evaluation, or the outcomes which should, or should not, be measured and reported5.
The work described here looked at the ‘Implications for research’ sections of reviews published by two Cochrane Review Groups between 2009 and 2019 (2019 was incomplete at the time of the work). Our aims were to:
We focused on the Schizophrenia Review Group and the Multiple sclerosis and rare diseases of the central nervous system Review Group (which we call the MS Review Group hereafter). The Schizophrenia Review Group was chosen because it has a long-standing interest in what is written in the ‘Implications for research’ section and we expected it to have good, consistent reporting. The MS group was selected because one of us (SP) had a special interest in this clinical area.
The work was split into two stages:
1. Identify and extract the ‘Implications for research’ sections of all reviews in both groups with a citation date of 2009 to 2019. All reviews are available at the following URLs:
1. Schizophrenia Review Group: https://schizophrenia.cochrane.org/topic-tree
2. MS Review Group: https://msrdcns.cochrane.org/our-review
2. Categorise the recommendations on trial design and methodology made by the review authors.
Stage 1 was straightforward because the ‘Implications for research’ sections of Cochrane reviews are easily identified. Stage 2 involved reading through the text and putting the issues raised by review authors into categories. We used a simple Excel spreadsheet data extraction form for this (see Data availability). We did not try to fit the data into pre-existing categories but were guided by what came from the data. The initial categorisation was done by SP before being discussed with ST until agreement was reached. Additionally we extracted information on the number of included participants and studies, the risk of bias etc. (See Data Availability for a file containing the full list). No statistical analysis was necessary beyond counting.
For the period 2009 to 2019, we identified 162 reviews for the Schizophrenia Review Group and 43 reviews for the MS Review Group. A wide variety of interventions were covered by these reviews, including drugs, educational and behavioural interventions, and other therapies such as physical exercise and acupuncture. The median number of included studies in reviews from the Schizophrenia Group was seven (range 0 to 174); for the MS Group it was five (range 0 to 45).
We created 22 categories in total, of which 12 were common to both review groups (Table 1). Six categories were unique to the Schizophrenia Review Group and four were unique to the MS Review Group. The five most used categories were the same for both groups (better choice of outcomes, better choice of intervention/comparator, longer follow-up, larger sample size and use of validated scales). However, the ranking of each category in that top five list varied from one year to the next; no category was consistently most used (Figure 1 and Figure 2). There was no obvious pattern of improvement over time for trials included in systematic reviews published by both groups.
A total of 22 categories were identified, 12 of which were common to both the Schizophrenia and Multiple Sclerosis (MS) Review Groups. The five most frequently occurring implications for research (marked in grey) were the same for both review groups.
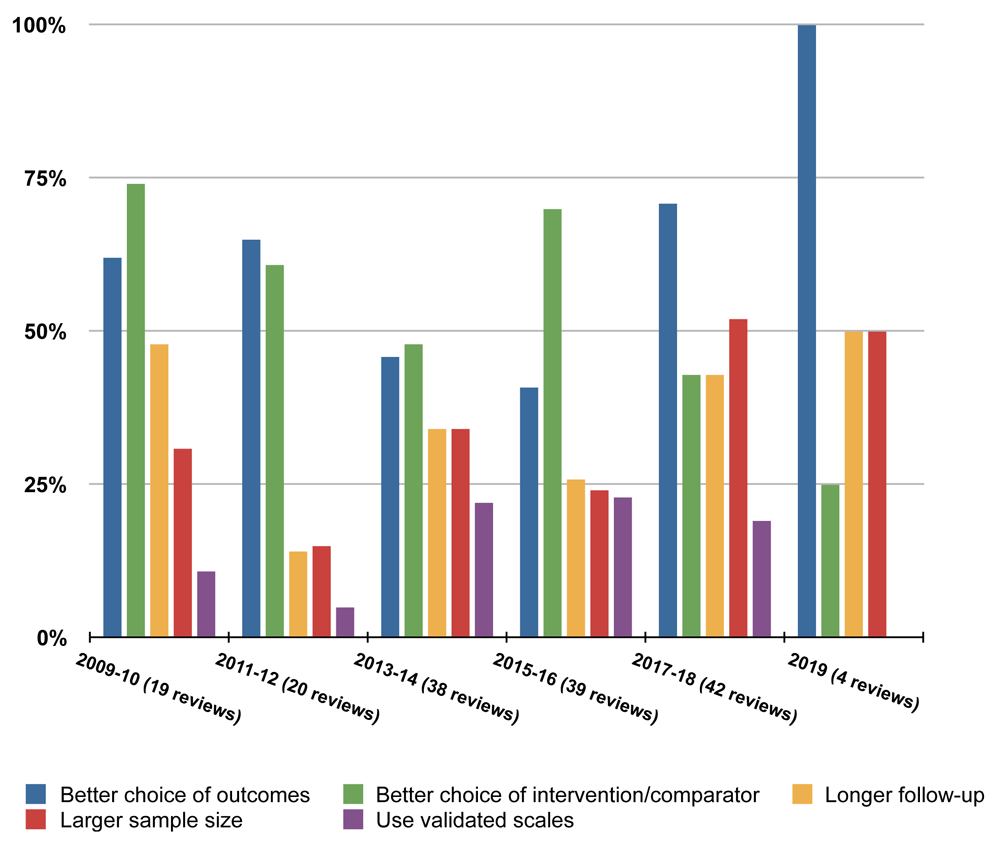
The percentages shown are the average over a two-year period, apart from 2019 which was incomplete at the time the work was done. While some categories are used in well over half of all review published in a given year, there is no clear pattern over time.

MS=Multiple sclerosis and rare diseases of the central nervous system. The percentages shown are the average over a two-year period, apart from 2019 which was incomplete at the time the work was done. While some categories are used in well over half of all reviews published in a given year, there is no clear pattern over time. Use of ‘Better choice of outcomes’ may be increasing but review numbers are small so caution is needed in interpreting this increase.
There is substantial overlap in the types of recommendation made in the ‘Implications for research’ sections of systematic reviews done by the Cochrane Schizophrenia and MS Review Groups. These were easier to identify in the Schizophrenia Review Group’s reviews because of their consistent approach to presenting implications for research in accordance with published guidance6. Many of their reviews also routinely include a suggested design for a future trial in this section.
The five most frequently made recommendations are the same for both groups with better choice of outcomes being top of the list and used in over half the 205 reviews included in our study. Looking across the decade to 2019, there is no obvious pattern of decrease in the areas of methodology that need to be improved in trials included in systematic reviews published by both groups.
Previous research found that small, underpowered studies make up the entirety of evidence in most meta-analyses reported by Cochrane reviews7. Only 35% of the reviews in our sample mentioned sample size in the Implication for research, which suggests that reviewers may be underestimating the size of this problem. The most frequent issue raised in our work was the choice of outcome, a persistent problem that led to the COMET initiative to facilitate the development of core outcome sets5,8. Cochrane reviewers need to continue to highlight trial design problems; indeed they could perhaps do so more often, especially around sample sizes.
It is possible that different researchers would have categorised the implications for research differently, although we did use two reviewers working independently and there was little disagreement. Selecting other Cochrane review groups may have led us to conclude that different recommendations were most common but without doing that work we have no way of knowing this.
Despite looking at just two Cochrane review groups, we believe that our findings are likely to be generalisable to other areas of health and health care but, at a minimum, a good start for the 2020s would be for researchers planning trials in schizophrenia and multiple sclerosis to ask themselves (or be compelled to do so by others, e.g. funders) the following questions:
1. Why did we choose these outcomes?
2. Why is this the right intervention and comparator?
3. Is follow-up long enough?
4. Is the sample size large enough?
5. Will validated scales be used to measure outcomes?
There are resources that can help with the above. For example COMET (http://www.comet-initiative.org) can help guide outcome choice and PRECIS-2 can help with design decisions around comparators and follow-up9. Discussions with staff at clinical trial units and other research support centres are also likely to improve designs. Change is slow at present and initiatives to encourage, or force, trialists to consider these questions would be welcome, especially from funders.
Open Science Framework: Data extracted from the Implications for Research sections of reviews from two Cochrane Review Groups. https://doi.org/10.17605/OSF.IO/XCJ7R
This project contains the following underlying data:
Open Science Framework: Data extraction form used in 'What improvements do Cochrane systematic reviewers suggest for the design of trials?'. https://doi.org/10.17605/OSF.IO/FNJVZ
This project contains the following extended data:
Open Science Framework: Full data extraction from the Implications for Research sections of reviews from two Cochrane Review Groups. https://doi.org/10.17605/OSF.IO/P9CW6
This project contains the following data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Methodological research, clinical epidemiology.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Evidence based clinical practice.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Methodological research, clinical epidemiology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 25 Jun 20 |
read | |
|
Version 1 11 Mar 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)