Keywords
Avicennia, cytotoxic, polyisoprenoids, colon cancer, mangrove
Avicennia, cytotoxic, polyisoprenoids, colon cancer, mangrove
We have revised in the part of Results and Discussion by adding new references no. 19, 33-34 to incorporated Reviewers' suggestions and to support our results.
See the authors' detailed response to the review by Ewa Swiezewska and Liliana Surmacz
See the authors' detailed response to the review by Aldes Lesbani
Cancer is a disease characterised by uncontrolled cell growth. Cancer cells can evade apoptosis and avoid signals that suppress its growth, impede the ability to form new blood vessels (angiogenesis), and spread its invasion and metastasis1. According to the Global Cancer Observatory, in 2018, Asia had the highest incidence of colon cancer with 51.8% of the global cases. Colon cancer is one of the top three causes of death in the world1. The use of chemotherapeutic agents constitutes a treatment for colon cancer, in addition to surgery and radiation therapy. Chemotherapeutic agents generally suppress the growth or proliferation of cancer cells, simultaneously causing toxicity in the body2.
Natural substances developed as potential chemotherapeutic agents include components of mangrove leaves. Mangroves are vegetation formations found in littoral areas in tropics and subtropics3. Polyisoprenoids are secondary metabolites found in several mangroves, distributed as dolichol and polyprenol on the leaves and roots of mangrove plants4. So far, few studies have reported the pharmacological activity of polyisoprenoids obtained from mangrove species. Thus, it is essential to study the potential and mechanisms of polyisoprenoids in mangroves as a natural ingredient for anticancer pharmaceuticals and medication4. For instance, methanol extracts of Avicennia alba (bark and leaves) present anti-proliferative activity in human breast cancer cell lines MCF-7 and T47D5. Additionally, this extract has cytotoxic effects on a variety of cancer cells, including colon cancer cells – HT-296. Previous research suggests that polyisoprenoids induce cancer cell cycle inhibition in adenocarcinoma of the colon (COLO 320 HSR, WiDr, and LS174 cells) in the G2-M phase and reduce the percentage of Bcl-2 and Bcl-xL7. Polyisoprenoids have been previously reported as chemopreventive agents for colon cancer8, given that polyisoprenoids in A. lanata leaves have displayed anticancer activity for the same9. On the other hand, A. alba contains polyisoprenoids that induce cell cycle, apoptosis, and gene expression of cycloogenase-2 (COX-2) in colon cancer cells WiDr10. Polyisoprenoids have a mechanism to inhibit the cell cycle at the G0-G1 phase, and apoptotic analysis occurs in the early phase of apoptosis in WiDr cells9,10.
The present study analysed the effect of immune-related genes’ expression on WiDr cells in vitro using reverse transcription-polymerase chain reaction (RT-PCR). RT-PCR was developed as an in vitro test to measure the biological activity of plasmid DNA-based products (pDNA). The said test measures RNA-specific transgenic messengers (mRNA) derived from transfected cultured cells. Forward and reverse primers have been designed to trigger selective RT-PCR reactions for plasmid mRNAs11. Therefore, the present study aims to investigate the inhibitory activity of polyisoprenoids obtained from the leaves of mangrove plants A. alba and A. lanata concerning the expression of PI3K, Akt1, mTOR, P53, and EGFR genes in human colorectal adenocarcinoma WiDr cells. The selection of the particular pro-apoptotic genes analysed, PI3K, Akt1, mTOR, and EGFR as the target of cancer therapy in the process of proliferation and induction of apoptosis, whereas the prevention of cancer formation involves a mechanism on the tumour suppressor protein p53.
The leaves of two mangrove species – A. alba and A. lanata – were collected from the village of Lubuk Kertang, Brandan Barat, Langkat, North Sumatra, Indonesia. The sample site is situated at 04° 07' 39.71'' North latitude and at 98° 30'97.87'' East longitude.
Five hundreds g of powdered mangrove leaves of A. alba and A. lanata was macerated with a mixture of chloroform/methanol (2:1, v/v) (CM21) for 48 h. Precipitate insoluble in CM21 was removed by filtration paper (Advantec, Japan) and the extract was partially purified as lipid extract. The lipid extracts of leaves were refluxed at a temperature of 65°C for 24 h in 86% ethanol containing KOH 2 M. The unsaponifiable lipid partitioned into 2 mg/mL n-hexane. The extract in n-hexane was concentrated using rotary evaporator at 40°C. Subsequently, a thick extract was obtained and the concentration was adjusted to 1 mg/mL n-hexane.
The unsonifiable lipid (50–100 mg) leaf extracts were analysed by silica gel 60 thin layer chromatography (TLC) and RP-18 high performance thin layer chromatography (HPTLC) plates (Merck) to identify the polyisoprenoid composition4,12,13. The polyisoprenoid standards were generously provided by Dr. Ewa Swiezewska (Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland). The quantity of polysioprenoids in A. alba leaves was 5.5±0.8 mg/g dry weigh and A. lanata leaves was 14.9±1.2 mg/g dry weight. Data are represented as the means ± SEM (n=3). The polyisoprenoid compounds in the A. alba (PAA) and A. lanata (PAL) leaves were detected to be 100% dolichol family with chain length of C60–C100 and C70–C100 in two dimensional TLC chromatogram (2D TLC), respectively4. No polyprenol was found in both mangrove leaves. The 2D TLC of both samples was performed triplicates and showed identical pattern, we confirmed that two mangrove leaves extracts contained 100% dolichols, therefore it is not required to purify the polysioprenoid samples and used for further investigation.
WiDr cells (isolated human colon cancer cells) and normal cells (Vero ATCC® CCL-81™, an immortalized cell line, derived from the kidney of an African green monkey) were kindly provided by the Laboratory of Parasitology Collection, Faculty of Medicine, Gadjah Mada University (Yogyakarta, Indonesia). The WiDr cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (RPMI powder sachet contained 2 mM L-glutamine without NaHCO3, 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), NaHCO3, 1 N HCl, 1 N NaoH, sterile water) (Sigma Aldrich, Singapore) and the Vero cell lines were nurtured in M199 (M199 powder sachet with Earle’s salt and 2mM L-glutamine without NaHCO3, 20 mM HEPES, 1 N HCl, 1 N NaOH, sterile water) (Gibco, USA). Both cells were supplemented with 10% (v/v) foetal bovine serum (FBS) (Gibco), 1% penicillin and streptomycin (Gibco), and 0.5% fungizone (amphoterin B) in a 37°C incubator with 5% CO211.
Unsaponifiable lipid of PAA and PAL were weighed 5 mg each in a microtube, and 50 μM of 5-fluorouracil (5-FU) were dissolved in a 100 μL dimethyl sulfoxide (DMSO: 0.05 %) co-solvent and vortexed for 10 min. The test solution was serially diluted with a concentration of 1000 µg/mL, 500 µg/mL, 250 µg/mL, 125 μg/mL, and 62.5 μg/mL. All sample dilution processes was carried out using the RPMI culture media supplemented with 10% (v/v) FBS, 2% penicillin-streptomycin, 0.5% amphotericin B (fungizone) for the WiDr cell test solution and M199 culture media (M199 medium, 10% FBS, 3% penicillin-streptomycin, and 1% amphotericin B (fungizone)) for Vero cell test.
Cytotoxicity tests were conducted on the WiDr and Vero cells using the MTT ((3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) method. Cells were grown in 96 well microplates to obtain a density of 1 × 104 cells/well and incubated in a 5% CO2 incubator at a temperature of 37°C for 48 h to ensure good growth. Once the new medium had been replaced, the cells were exposed with serially diluted concentrations of PAA and PAL (1000, 500, 250, 125, and 62.5 µg/mL) in the cell cycle analysis as previously reported10. 5-FU (Sigma Aldrich) was used as a positive control with the same concentration of PAA and PAL and incubated in 5% CO2 at 37°C for 48 h. At the end of the incubation, the culture media was removed, and the cells were washed with PBS. In each of the wells, 100 mL of culture medium (RPMI) and 10 mL MTT (Sigma Aldrich) were added. The cells were incubated again for 3–6 h in 5% CO2 at 37°C. The reaction was stopped with 10% SDS reagent (Sigma Aldrich) in 0.01 N HCl (Merck). The plate was wrapped to protect it from the light so that the wells were opaque, and it was left overnight at room temperature14. Absorption was measured by the ELISA reader (Benchmark 10431, BioRad) at a wavelength of 595 nm.
Selectivity index (SI) was determined from the IC50 of the polyisoprenoid extract from PAA and PAL leaves in Vero cells versus WiDr cells to exhibit the cytotoxic selectivity of the polyisoprenoid extract, as previously reported9. IC50 was calculated from concentrations that caused death among 50% of the cell population analysed using probit analysis in SPSS version 23 with a significance of 0.058.
The WiDr cells (5 × 103 cells/well) were added to a 6-well plate which was incubated for 24 h for optimal growth. Subsequently, the cells were exposed to selected concentrations of PAL and PAA (1/5 IC50) and incubated again10. Floating as well as attached cells were collected by adding 0.025% trypsin. The cells from each well were transferred to a separate eppendorf tube. 1 mL PBS was added and the PBS was removed with a micropipette and centrifuged at 2500 rpm for 5 min. The supernatant was removed and 1 µL RNase/PI staining solution (Thermo Fisher Scientific) was added and kept for 10 min a dark place (avoiding light) at 37°C. The cell cycle distribution was analysed using the FAC Scan Flow Cytometer (BD Biosciences) and the percentage of cells obtained in each cell cycle phase (G1-S and G2-M) was calculated using the software ModFit LT. 3.0 s for Windows (Verity Software House).
The double staining method was used to determine the level of apoptosis. WiDr cells were grown in a 6-well microplate at density of 5 × 105 cells/well and incubated for 24 h. The following day, the cells were treated with concentrations of PAA and PAL (1/5 IC50) and incubated for 24 h. The procedure for apoptosis with double staining was conducted by taking cells from the CO2 incubator and observing the conditions. Then, the calculated cells were used to prepare 24-well plates and slipcovers. 200 µl of cell suspension was evenly and slowly transferred just above the coverslip. The cell was kept in the incubator for 3–30 min to attach to the coverslip with 800 μl of culture media which was added for 48 h of incubation. The culture media was slowly disposed, and the cells were washed with PBS (500 µl). The sample and media were added into the well for control cells and then incubated. All media from the well was slowly removed using a Pasteur pipette. Cells in the wells were washed with PBS. The coverslip was removed using tweezers, placed on a glass slide, and labelled. 10 μl of the reagent mixture of ethidium bromide acridine orange (Sigma Aldrich) was added over the slipcover. The mixture was flattened and gently rocked. Apoptosis was observed under a fluorescence microscope (Olympus CKX41)9. ImageRaster 4.0.5 (Miconos, Yogyakarta, Indonesia) was used to count green and red fluorescence signals of three microscopic snapshots of individual experiments. The fluorescent green cells were alive and the fluorescent red cells were dead15. Red fluorescent intact cells indicated necrotic cells while fragmented cells indicated apoptotic cells.
Total RNA was extracted from the WiDr cells treated with PAA and PAL (7.5 × 108 cells/well) using the Total RNA Mini Kit (Geneaid), according to the manufacturer’s protocol. The total RNA (0.3 µg each) was reverse-transcribed with 1 µg random primer to produce cDNA in a total volume of 20 µl using ReverTra Ace kit (Toyobo) with 10 mM dNTP to incubate for 10 min at 30°C, for 60 min at 42°C, and for 5 min at 99°C according to manufacturer’s procedure. The resulting cDNA mixture was diluted using 100 μL TE buffer (10 mM Tris/HCl, 1 mM EDTA, ph 8.0) and directly used for the subsequent PCR.
Semi-quantitative RT-PCR for genes p53, EGFR, PI3K, Akt1, and mTOR16–21 were assessed using 1 μL cDNA added to 25 μL PCR Master Mix which contained 12.5 μL GoTaxGreen, 1 μL primer forward, and 1 μL primer reverse (as listed in Table 1), and 9.5 μL DNase/RNase free water. 35–40 cycles of semi-quantitative RT-PCR (ProFlex PCR system, Thermo Fisher Scientific) were conducted under the following cycling conditions: 15–30 sec at 94°C, 45 sec at 94°C, and 10 sec at 55–60°C, with the final extension phase at 72°C for 5 min and then storage at -20°C22. Semi-quantitative RT-PCR products were observed using 2% agarose gel and stained with ethidium bromide. The bands were documented using the image scanner Doc XR Gel (Bio-Rad)23. Each data represents the average of three independents RT-PCR measurements with standard errors of individual experiments. To quantify the PCR product, Quantity One® 1-D analysis software (Bio-Rad) used to assess bands intensity of genes analysed. β-actin was reference gene to normalize the PCR efficiency.
All the data were analysed using SPSS version 23, followed by Duncan’s multiple range test for treatment comparisons. The data are presented as mean ± standard error of the mean (SEM). One-way variance analysis (ANOVA) was used to compare the results for different conditions. P < 0.05 was considered significantly different.
The cytotoxicity test is a preliminary parameter to determine the potential toxicity of a test substance, particularly cancer cells. The toxicity is expressed by IC50 parameters. However, the cytotoxic test can also be performed to assess the toxicity of a test substance on normal cells. So, it can be used to demonstrate selective cytotoxic effects against a cancer line. In this study, the cytotoxic test material was derived from polyisoprenoids of mangrove leaves (PAA and PAL) against colon cancer cells (WiDr) with a concentration series of 1000, 500, 250, 125, and 62.5 μg/mL. The purpose of testing the extract was obtaining the smallest IC50 value for subsequent use as an advanced test for anticancer activity. The results showed that the smallest IC50 value obtained from the polyisoprenoids of leaves from PAL was 243.32 μg/mL and from PAA was 258.14 ug/mL. Therefore, these concentrations were used in the remaining experiments of the study. The cytotoxic effects were indicated by absorbance values and analysed using a probit analysis to obtain IC50, as shown in Table 2.
SI: selectivity index.
The greatest cytotoxic activity against WiDr cells, shown by the smallest IC50 value, was obtained from PAL. Therefore, PAL has the most active anticancer activity because the IC50 value showed that PAL could block 50% of the WiDr cell growth. Some extracts are only considered active if they have IC50 values ≤100 μg/mL16. However, it has been demonstrated that an extract value of IC50 of 100–500 μg/mL can be classified as moderate and, therefore, can potentially be developed as an anticancer agent17. Even though a study has reported that an extract is considered active if IC50 > 500 μg/mL18. As shown in Table 2, the SI of PAL and PAA were lower than that of the positive control. The cytotoxicity of PAL and PAA in the present study included an interesting SI against WiDr cells in a dose-dependent manner17, suggesting the higher cytotoxic selectivity (i.e. safety) of polyisoprenoid extracts against normal than cancer cells. The previous study has reported that mangrove genus of Avicennia have cytotoxicity effects against several cancer cell lines (HL-60, HepG2, NCI-H23) utilize MTT assay method19.
A previous study showed that methanol and water extracts of A. alba leaves have distinctive properties in regulators and mediators of cancer20. This study tested the cell cycle using flow cytometry to determine the distribution of cells in each phase of the cell cycle at sub G1, S, and G2-M after treatment and obtained predictable pathway inhibition using PAA and PAL to inhibit the cycle cell21.
The inhibition of the cell cycle in this study is shown in Figure 1 and Table 3. Table 3 shows the control group’s WiDr cell accumulation in the G0-G1, S, and G2-M phase as 76.63%, 7.22%, and 17.93%, respectively. The accumulation of cells in the S phase and G2-M cells increased by 10.60%, 10.51% and 23.84%, and 22.05%, respectively after being administered with a concentration of PAL 1/5 IC50 and PAA with the concentration of 1/5 IC50. The phase change is considered related to the concentration. However, the overall mechanism of inhibition of the cell cycle for PAA and PAL occurred at S and G2-M phases.
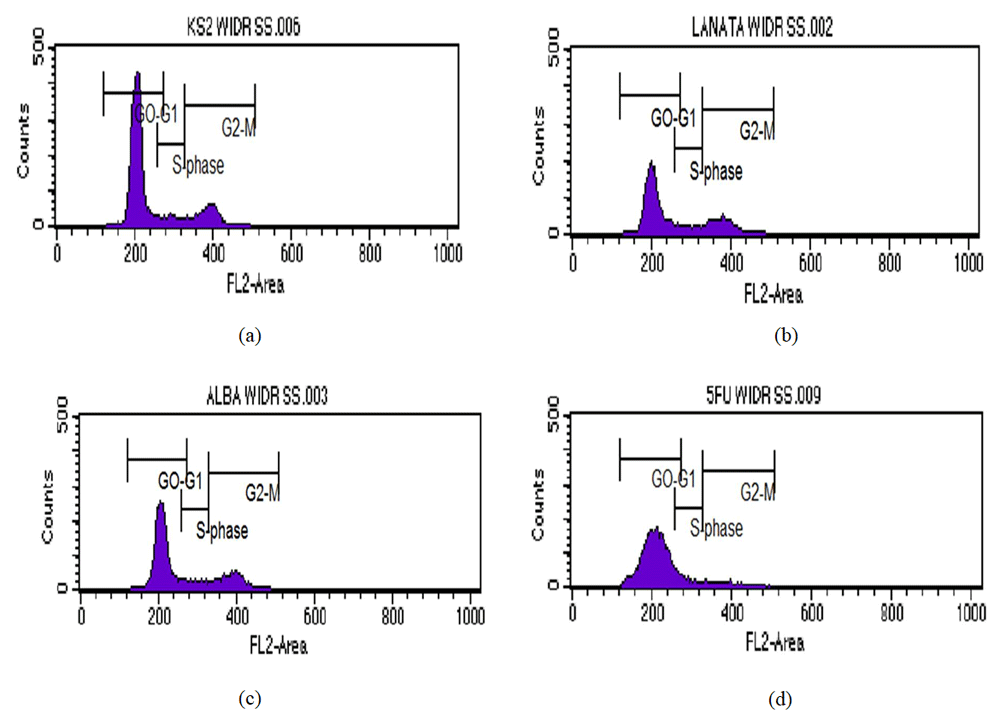
Cell cycle analysis showing the percentage accumulated in each phase of the cell cycle WiDr after the administration of the extract of mangrove leaves and 5-FU as a positive control: (a) Control cell; (b) PAL 1/5 IC50; (c) PAA1/5IC50; and (d) 5-FU1/5 IC50.
| Treatment | Concentrations (μg/mL) | Phase of the cell cycle (%) | ||
|---|---|---|---|---|
| G0-G1 | S | G2-M | ||
| Control cell | - | 76.63 | 7.22 | 17.93 |
| PAL 1/5 IC50 | 50 | 68.70 | 10.60 | 23.84 |
| PAA 1/5 IC50 | 52 | 70.39 | 10.51 | 22.05 |
| 5-FU 1/5 IC50 | 3.6 | 88.12 | 9.52 | 6.42 |
As Table 3 illustrates, the administration of 5-FU with 1/5 concentration IC50 decreases the accumulation of WiDr cells in the G2-M phase at 6.42%. The increase in cell accumulation occurred in the G0-G1 phase – S was 88.12 and 9.52%. However, it can be confirmed that the overall mechanisms of cell cycle inhibition of 5-FU (in the G0-G1 and S phases) had a different mechanism compared with PAL and PAA. Treatment of cancer cells with 5-FU can accumulate cells at the G1 phase and at the beginning of the synthesis phase (G1-S arrest)22. However, the cell cycle’s inhibitory activity using 5-FU depends on the type of cancer cell. In colon cancer cells HCT-15 and HT-29, 5-FU inhibited at the G2-M phase. 5-FU increases the expression of cyclin A, cyclin B, and CDC2, which is a regulatory protein in the G2-M phase23. The mechanism that mediates the activity in this phase needs to be explored further. In Lovo and WiDr cells, 5-FU inhibits the cell cycle in the S phase18. This suggests that the activity of 5-FU is not always associated with thymidylate synthase inhibitory activity, and the activity of 5-FU in the cell cycle if used in a different cell needs to be researched further.
In the present study, increased apoptosis (seen using the reagent acridine orange-ethidium bromide through the fluorescence microscope) was obtained by a percentage increase in each of the phases. Apoptosis is generally characterised by different morphological characteristics and biochemical mechanisms that depend on energy24. Apoptosis usually occurs during development and aging and as a homeostatic mechanism to maintain the population of cells in the network. It also is a defence mechanism when cells are damaged by disease or harmful agents or during immune reaction25. Cytotoxic data test samples indicate the presence of a cytotoxic effect as shown in Figure 2. The green cells are the live ones while the red ones are dead. The range of red fluorescent cells represents the necrotic cells.
The present analysis used ImageRaster to count the dead cells and control cells after observing them under a fluorescence microscope. In average, 96.21% green colour (living cells) and 3.79% red colour (dead cells) were observed in control cells with three independent experiments. With the PAL 1/5 IC50 treatment, the cells produce 65.66% green colour (living cells) and 34.34% red (dead cells). With PAA 1/5 IC50 treatment, cells produce 72.42% green colour (living cells) and nearly 27.58% red colour (dead cell). With 5-FU 1/5 IC50 treatment, the cells produce 82.46% green (live cells) and 17.53% red colour (dead cells). There was no statistically significant difference among the treatments (Figure 2).
The results of the control cells were observed to be green/alive cells. The green colour comes from the orange acridine penetrating the entire living cell with intact membranes and nuclei. In cells treated with PAL and PAA, there was a predominantly red colour which illustrates that the WiDr cells were dead. The orange colour is produced by ethidium bromide interacting with damaged cell membranes and nuclei26. The test results showed that the extract can inhibit the growth of cancer cells, especially in WiDr cancer cells. The inhibition capability through the mechanism of apoptosis can also be evidenced through testing and analysis of double staining flow cytometry. The results of both analyses can illustrate the mechanism of cell death caused by apoptosis both quantitatively and qualitatively. Thus, the induction of apoptosis shows that these treatments are a promising treatment for cancer. The cancer cells undergo apoptosis and lose their ability to proliferate rapidly. This way of treatment may induce usual apoptotic signalling, thereby, potentially eliminating the cancer cells27.
The potential working mechanism of PAL and PAA are in the late phase of apoptosis. The potency of PAL and PAA in triggering apoptosis may be caused by compound isoprenoids (based on the results of phytochemical screening of PAL and PAA). Steroids/triterpenoids are compounds that have high anticancer activity, by blocking nuclear factor-kappa B, inducing apoptosis, and activating transcription and angiogenesis, which can be useful in the treatment of various types of cancer28.
The measurement of the expression of PI3K, Akt1, mTOR, P53, and EGFR genes using RT-PCR produces the band illustrated in Figure 3. Gene expression level was quantified using a computerised system (Table 4). Table 4 shows significant differences between the treatment groups. Gene expression results on PI3K, Akt1, mTOR, P53, and EGFR differed significantly (p < 0.05).
In this study, RT-PCR showed that the anti-apoptotic gene expression of P53 increases compared to control, whereas the expression of pro-apoptotic genes (PI3K, Akt1, mTOR, and EGFR) tend to decrease. P53 is a tumour-suppressing protein that can affect the permeability of the mitochondrial membrane and directly induce apoptosis without inducing the transcription of the target gene associated with apoptosis in advance29. This condition causes apoptosis when the gene Bax mRNA expression does not increase and, thus, the pathway of apoptosis by P53 has two paths to the mitochondria – directly and indirectly through the activation of the transcription of genes under it. P53 molecule is a tumour-suppressing protein found at a low level under normal conditions and has a short life span. P53 is activated when the cells are exposed to stimuli such as agents that cause DNA damage, hypoxia, lack of nucleotide, or tumour cell activation. As a tumour suppressor, P53 protects the genome and regulates the growth and proliferation of the critical points in response to stress. The P53 molecule is an upstream regulator of the cell cycle as well as the intrinsic apoptotic pathway mediated by the Bcl-2 protein30.
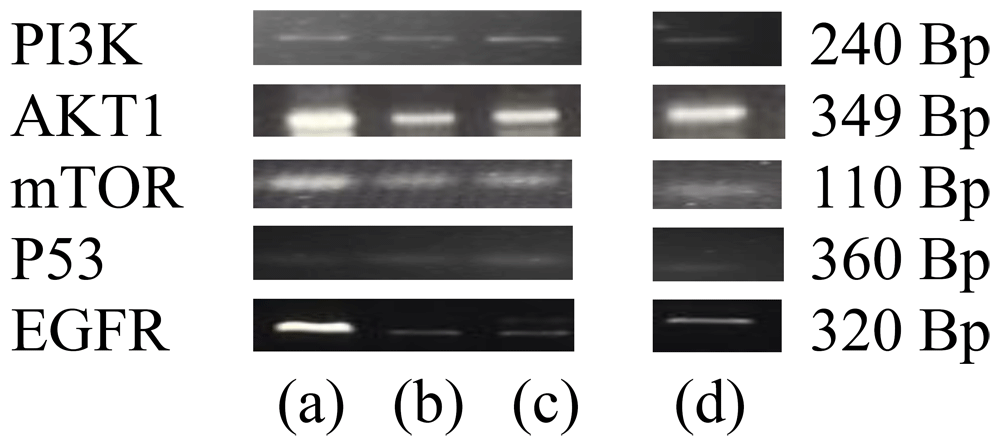
(a) control cell; (b) PAL; (c) PAA; (d) 5-FU.
| No | Gene | Treatment group | Expression level average value ± SEM |
|---|---|---|---|
| 1 | PI3K | Control cell | 1 ± 0.00bcd |
| PAL | 0.76 ± 0.01acd | ||
| PAA | 0.85 ± 0.01abd | ||
| 5-FU | 0.95 ± 0.01abc | ||
| 2 | Akt1 | Control cell | 1 ± 0.00bcd |
| PAL | 0.54 ± 0.01acd | ||
| PAA | 0.86 ± 0.01abd | ||
| 5-FU | 0.95 ± 0.01abc | ||
| 3 | mTOR | Control cell | 1 ± 0.00bcd |
| PAL | 0.84 ± 0.01acd | ||
| PAA | 0.76 ± 0.01abd | ||
| 5-FU | 0.72 ± 0.01abc | ||
| 4 | P53 | Control cell | 1 ± 0.00bcd |
| PAL | 3.03 ± 0.01acd | ||
| PAA | 2.94 ± 0.01abd | ||
| 5-FU | 2,27 ± 0,01abc | ||
| 5 | EGFR | Control cell | 1 ± 0,00bcd |
| PAL | 0.19 ± 0.01acd | ||
| PAA | 0.11 ± 0.01abd | ||
| 5-FU | 0.04 ± 0.01abc |
A statistically significant followed by Duncan’s test
a. (P) < 0.05: There is a significant difference with the normal group (control cell).
b. Sig (P) < 0.05: There is a significant difference with the group PAL.
c. Sig (P) < 0.05: There is a significant difference with the group PAA.
d. Sig (P) < 0.05: There was no significant difference in the positive control group (5-FU).
Besides from being a tumour suppressor protein, P53 also acts as a transcription factor for the activation of the expression of multiple target genes involved in various biological functions, such as apoptosis and cell cycle arrest31. The results of the present study show that PAL and PAA significantly increase the gene expression of P53 comparing to control cell and 5-FU. These results are consistent with previous studies stating that the expression of P53 protein increases apoptosis32.
The level of PI3K, Akt1, EGFR and mTOR gene expression was been significantly downregulated in treatment cells compared to the control cell in the present study. PAL administration demonstrated a more significant reduction in PI3K, Akt1 and EGFR gene expression than PAA and 5-FU, while mTOR was downregulated more with 5-FU than PAL and PAA. The level of the P53 gene expression was significantly upregulated in the treatment cells compared to the control cell, and this was more significant with PAL than PAA and 5-FU. In this circumstance, the level transcripts may be directly correlated to the activity of protein kinases such as polyisoprenoids decreased the level expression of PI3K, Akt1, and mTOR genes to its function in the cell.
In the present study, polysioprenoids showed anticancer activity in WiDr cells through the effect of the tumour suppressor and the pro-apoptotic gene expression. This study also indicated that mangrove polyisoprenoids blocked the development of WiDr colon cancer. Furthermore, our previous studies have shown that polyisoprenoids from mangrove leaves induced apoptosis, decreased cell proliferation, and exhibited anticancer activity8–10. We on the basis of present and previous studies proposed mechanism of inhibition of cell cycle at G0-G1 phase and enabled the suppression of COX-2 and p53 against WiDr colon cells. Based on the previous investigation results showed that isolated compound from mangrove genus of Avicennia have activities for induce apoptosis by the regulation of apoptotic-related genes (p53 and Bcl-2 pathways) on cancer cell line (i.e. MDA-MB 231 cells) utilize two-step RT-real time PCR method33. This postulate represents a new mechanism of mangrove polyisoprenoids to exhibit anticancer activity and appeared to merit further investigation.
5-fluorouracil (5-FU) has been widely used to treat colon cancer since 1957; however in the long term, it can have toxic effects or resistance to effectiveness. Cell cycle perturbation has been reported as one of the causes to lead 5-FU resistance34. Therefore, need to seeking the better therapeutic strategies for potential anticancer drugs identified from alternative sources, including natural products e.g. mangrove.
Overall, the present study confirmed that PAL and PAA can affect anti-apoptotic P53 gene expression by upregulating this gene than the controls, while the expression of pro-apoptotic genes PI3K, Akt1, mTOR, and EGFR were downregulated compared to the controls. In addition, PAL and PAA inhibited the WiDr cell cycle in later apoptosis (S and G2-M1). Therefore, this study confirms that the polyisoprenoids derived from A. alba and A. lanata leaves are promising chemopreventive agents and facilitate the potential usefulness of polyisoprenoids as new drugs for colon cancer.
Figshare: Dataset for manuscript: Effects of polyisoprenoids from Avicennia lanata and Avicennia alba leaves on the gene expression of P13K, Akt1, mTOR, P53, and EGFR in human colorectal adenocarcinoma WiDr cells using reverse transcription-PCR, https://doi.org/10.6084/m9.figshare.11839350.v435.
This project contains the following data:
Figshare: Dataset for manuscript: Effects of polyisoprenoids from Avicennia lanata and Avicennia alba leaves on the gene expression of P13K, Akt1, mTOR, P53, and EGFR in human colorectal adenocarcinoma WiDr cells using reverse transcription-PCR, https://doi.org/10.6084/m9.figshare.11856039.v436.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Genetic Natural Product.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Genetic Natural Product.
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 4 (revision) 09 Dec 20 |
read | ||
|
Version 3 (revision) 26 Oct 20 |
read | ||
|
Version 2 (revision) 24 Apr 20 |
read | read | |
|
Version 1 11 Mar 20 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)