Keywords
Vitex pinnata, antimicrobial, antioxidant, wound healing, wound excision
Vitex pinnata, antimicrobial, antioxidant, wound healing, wound excision
All changes have been made in response to reviewers 1 and 2. The Introduction has been revised to include several points to address the problem statement to include the importance of this research study. The Methodology has been clarified such as additions of chemicals and reagents used in the study, reference number of voucher specimens. The Results section has also been improved by the additions suggested by reviewers. We have also updated Figure 3 as there was an error with the error bars in the previous version. Labels are now included in Figure 4.
See the authors' detailed response to the review by Fitmawati Fitmawati
See the authors' detailed response to the review by Yoke Keong Yong
Vitex pinnata is a common ethnomedicinal plant locally known as ‘kulimpapa’ in Brunei Darussalam and East Malaysia. According to Goh et al. (2017), it is traditionally used as a treatment for stomach aches, fever, body aches and lesions. The bark may be used for abdominal pain, while the young shoots are utilized as sanitizing agents and deodorants. The roots and bark of this plant have been used in herbal baths and also taken orally in the form of decoction or herbal tea while the leaves may be eaten raw. Traditionally, the leaves were prepared into poultice and applied to wounds to induce fast healing (Burkill et al., 1966; Goh et al., 2017; Sahu & Barik, 2007; Suksamrarn & Sommechai, 1993). Although V. pinnata is a popular ethnomedicinal plant, scientific studies are lacking to validate its pharmacological properties. It is not clear if this plant has antioxidant, antimicrobial and wound healing properties.
Plants have been known to be a potent source of medicinal compounds and anti-oxidants. Oxidative stress produced through a chain reaction by free radicals can be a contributing factor to the pathophysiology of various conditions including cardiovascular dysfunction, atherosclerosis, inflammation, carcinogenesis, reperfusion injury and neurodegenerative diseases (Aruoma, 1998). Due to the increasing safety concerns with the consumption of synthetic antioxidants, alternative sources of antioxidants from natural origins, especially from plants, are currently in demand (Stankovic et al., 2016). This study is interested to determine if V. pinnata could be a good source of antioxidants.
Although various antibacterial agents of synthetic origin are available, bacterial resistance to current antibacterial agents is growing (Andersson & Hughes, 2010). Furthermore, available antibiotics can also cause side effects. For this purpose, plants can be good sources of antimicrobial agents due to their secondary metabolites (Nascimento et al., 2000). Previous studies have reported the antifungal activity of V. pinnata (Ata et al., 2009), however its antibacterial and antioxidant activity has not yet been reported. Therefore, this study also aims to screen its antibacterial activity against several bacterial strains.
In order to understand the possible effects of Vitex pinnata on wound healing, we have sought the use of animal models to reflect human wound healing conditions. Wistar rats were selected in our study because of their availability. In addition to this, the use of Wistar rats in this study provides a more mainstream model for understanding the wound healing process upon treatment with the extract. The use of this species and excision wound model also allows us to make a comparison of the wound healing process with other available literature involving a similar approach to ours. The scientific objective of this study is to determine the stage by stage process of wound healing such as general wound morphology, wound closure and presence of inflammatory cells, which could only be carried out in an animal model.
In this study, we report the antibacterial, antioxidant and wound healing activities of V. pinnata from Brunei Darussalam.
Ethyl acetate, chloroform, hexane, methanol and Xylene were purchased from Merck, Germany. DPPH (2,2-diphenyl-1-picrylhydrazyl), quercetin and streptomycin sulphate were from Sigma-Aldrich, Germany. Nutrient broth and Mueller-Hinton agar (MHA) were from Oxoid, United Kingdom. Diethyl ether was obtained from Emsure®, Germany. Formaldehyde was obtained from Scharlau, Spain. Paraffin wax was from Thermo scientific, United Kingdom. Hematoxylin was obtained from Fluka, Switzerland.
The leaves of V. pinnata were collected at the Universiti Brunei Darussalam Botanical Research Centre (UBD BRC) in Brunei Darussalam. Species identification was kindly carried out by the botanist at the UBD BRC. Voucher specimen of V. pinnata (catalog ID S00059) is available in UBD Herbarium (http://ubdherbarium.fos.ubd.edu.bn/). The plant samples were shade dried for a few weeks and grinded prior to solvent extraction.
A mass of 300 g of the ground leaves was exhaustively extracted with 1.5 L of methanol using Soxhlet extraction. The resulting methanol extract was then vacuum filtered and evaporated using a rotary evaporator. The methanol extract was further partitioned with different solvents in increasing order of polarity i.e. hexane, chloroform and ethyl acetate. A total of 10 g of the methanol extract was redissolved in approximately 1 L of methanol and 10 mL of distilled water, followed by the addition of 500 mL of hexane. After shaking vigorously, the mixture was left to stand to allow layers to form between the solvents. Once formed, the hexane layer was collected and the procedure was repeated with the other solvents (chloroform and ethyl acetate). Each solvent was evaporated with a rotary evaporator and subsequently air dried in a fume hood.
The percentage yield of extract was determined using the formula: Extraction yield (%) = [Dry weight of extract obtained / Dry weight of material used for extraction] × 100%. Prior to the antioxidant and antibacterial tests, each extract was re-dissolved in methanol at 1000 μg/mL and 500 mg/mL, respectively.
The antioxidant or radical scavenging activity (RSA) was determined by DPPH radical scavenging assay according to Awang-Jamil et al. (2019) with minor modifications. Each extract with a volume of 0.2 mL was mixed with 1 mL of 40 μg/mL of DPPH methanolic solution. For the control, 0.2 mL of methanol was used instead of the extract. After about 30 minutes, the RSA was determined by measuring the absorbance at 517 nm using a UV spectrophotometer. The assay was carried out in triplicate. The RSA (measured as the percentage of DPPH scavenged) was calculated using the formula: RSA (%) = [Acontrol – Asample]–/Acontrol × 100%, where Acontrol refers to the absorbance of the control and Asample refers to the absorbance of the extract.
To measure the quercetin equivalent (QE) concentration of each extract, the RSA of quercetin at six different concentrations (1, 5, 10, 20, 25 and 35 µg/mL) were also similarly measured. A standard calibration curve was prepared by plotting RSA (y-axis) against quercetin concentration (x-axis). Subsequently, the linear regression of the standard calibration curve (y = 2.74x + 2.77; R2 = 0.997) was employed for the estimation of QE concentration.
The microorganisms used in this study consisted of four different strains of bacteria: two Gram-positive bacteria, Staphylococcus aureus (ATCC-29213) and Bacillus subtilis (ATCC-11774); and two Gram-negative bacteria, Escherichia coli (ATCC-11775) and Pseudomonas aeruginosa (ATCC-27853). Each bacterial strain was grown in nutrient broth, and prior to the test, overnight bacterial culture was diluted to an absorbance of 0.08 to 0.1 (equivalent to 0.5 McFarland standard), measured with a UV spectrophotometer at 600 nm.
Antibacterial activities of the extracts were determined by disc diffusion method according to Abdullah et al. (2019) with minor modifications. A volume of 10 µl of each extract was applied onto a filter paper disc (6 mm in diameter) and left to dry. Methanol was used as the negative control and 20 mg/ml streptomycin sulphate (antibiotic) as the positive control. Each standardised bacterial culture was then swabbed onto Mueller-Hinton agar (MHA) plates. The impregnated discs were then loaded onto the swabbed MHA plates. The zone of inhibition was recorded after incubating the plates at 37 °C for 24 hours. Each test was carried out with at least three replicates.
Ethical statement. All work involving animals was approved by the Universiti Brunei Darussalam University Research Ethics Committee [approval reference: UBD/FOS/E2(g)]. All animal procedures were performed in accordance with Universiti Brunei Darussalam guidelines on the care and use of animals for research and internationally accepted guidelines. All investigators declare that every effort was taken to ameliorate harm to the animals via close monitoring for signs of pain and distress, and attended to in case signs of pain and distress was observed in accordance with the Universiti Brunei Darussalam guideline on care and use of animals for research.
Animals. Male Wistar rats aged between 8–10 weeks and weight range between 150–200g were used for the wound healing study. The animals were obtained from and housed in the Universiti Brunei Darussalam animal facility. The rats were fed with a standard maintenance pellet diet (Cat # 1324, Altromin, Germany) and access to food and water was provided ad libitum. Animals were housed in cages with wood shavings as bedding and maintained under standard lab conditions (12/12h light dark cycle; 25°–30°C). Prior to carrying out the experimental procedures, animals were assessed for their general health, determined by their behavioral responses to the handler and other general parameters such as hands-on physical examination, condition of the animal’s body, physical and observable abnormalities. The welfare of the animals was attended to throughout the experiments such as by the provision of food and water, avoidance by the handler of conditions that cause suffering, pain and disease. Animals with obvious signs of pain and distress during the experiment would be excluded from the study.
Control animals were placed between three to four animals per cage and following the wound excision procedure, animals were placed in individual cages throughout the duration of the experiment. Upon completion of the experiments, animals were euthanized using CO2 asphyxiation followed by cervical dislocation prior to tissue collections for histological analysis.
Formulation of ointment. The methanolic leaf extracts of V. pinnata were formulated into ointments with pure petroletum jelly as the base. Herbal ointments were prepared in two doses, which is the low dose extract consisting of 10% (w/w) extract and high dose extract containing 50% (w/w) extract. The maximum concentration that was stable in the base was 50% (w/w).
Excision wound. All procedures were refined in order to minimize any negative impact and pain to the animals resulting from the excision wound procedure. All procedures were carried out in the Universiti Brunei Darussalam animal facility at the same time of day between 10.00–13.00hrs. Prior to making the excision wound, all treated animals were anaesthetized using diethyl ether by inhalation anesthesia. Diethyl ether was chosen as the anesthetic because its action is also accompanied by analgesic properties. Animals were exposed to one drop of diethyl ether placed in a cotton wool and placed at one end of a conical tube. There was no close or direct contact between the cotton ball and the nose of the animal. Depth of anesthesia following exposure to diethyl ether was determined by responses to reflexes such as voluntary movements from stimuli such as extending of legs. Once deep anesthesia has been established, the animal excision wounds were performed as previously described (Umachigi et al., 2008). The dorsal fur of the animals was shaved and an area of about 100mm2 (10 × 10 mm) was excised using a sharp scalpel with disposable steel blades. Rats were then individually housed and the extract and ointment were applied topically on the wound area at the same time of day (between 10am –1pm) in the respective groups for a period of 21 days.
Animal grouping and dosing. Animals were randomly grouped into three groups, each group containing six rats. The numbers of animals (n) used in these experiments were based on previous studies in our laboratory and experiments of this nature (Nagar et al., 2016) and is also the minimum that is required to detect any significant difference between the treatment groups. Simple randomization was carried out when assigning the animals to groups in order to avoid any bias. Introduction of bias is reduced or eliminated as during the early stages of the experiment; i.e. during grouping, allocation of animals to treated and control groups was carried out randomly. Wound area determination was carried out by a validated instrument (Image J software, National Institute of Health, USA) which minimizes subjectivity of observer during assessment of wound areas.
Following this, all animals within each group received the same treatment throughout the duration of the experiment. The rats of Group I were treated with pure petroletum jelly only (negative control). Group 2 and Group 3 were treated with ointments containing 10% (w/w) leaf extract (low dose) and 50% (w/w) leaf extract (high dose), respectively. The ointment was topically applied to the wounds on alternate days for a period of three weeks.
Wound area determination. Wound area was measured using Image J software (National Institute Health, USA) (Figure 1). The rate of wound contraction was measured as the percentage of reduction of wound size. The percentage of wound contractions were calculated as previously described (Shi et al., 2013): Wound contraction (%) = 100 × [(Original wound area – area on measured post-wounding day) / Original wound area]
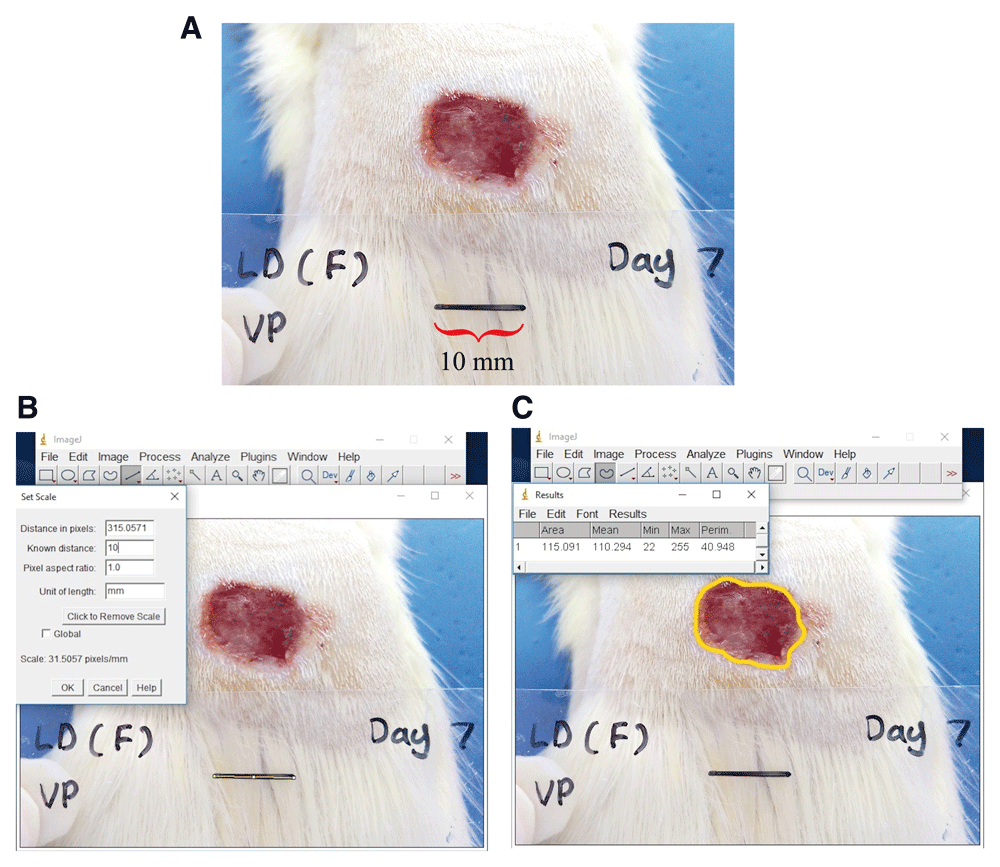
A) Anaesthetized rat with the scale placed near the wound site, labelled with name, post-wounding day and scale bar of 10 mm for measurement analysis. B) Calibration of Image J with known distance in image. C) The outline of the wound is drawn to measure wound area.
Histological analysis. Skin tissue samples from the wound and its vicinity were taken for histopathological analysis at post wounding days 3, 7, 14 and 21. Sections at 10µm thickness were prepared and stained with hematoxylin and visualized.
Soxhlet extraction yielded 8.0% of methanol extract from 300 g of ground leaves. For solvent partitioning, 20.3% hexane, 62.1% chloroform and 8.9% ethyl acetate extracts were obtained from 10 g of methanol extract.
Statistical analysis indicated that the RSA of the different extracts were significantly different to each other. The ethyl acetate extract had the highest RSA and QE concentration, indicating it was the most potent in inhibiting free radicals compared to the other extracts (Table 1). This was followed by the methanol extract, the chloroform extract and finally the hexane extract.
| Extract | RSA (%) | QE concentration (µg/mL) |
|---|---|---|
| Ethyl acetate | 93.6 ± 0.2 | 33.1 ± 0.1 |
| Methanol | 87.8 ± 0.5 | 31 ± 0.2 |
| Chloroform | 58.5 ± 1.5 | 20.3 ± 0.6 |
| Hexane | 15.1 ± 1.4 | 4.5 ± 0.5 |
Different solvents used for extraction have been reported to extract different compounds (Basri et al., 2017). It has been previously reported that phenolic content increased with the increasing polarity of solvent (Barchan et al., 2014; Belyagoubi et al., 2016). The extracts from V. pinnata in the present study possibly contained different types of phenolic compounds with different antioxidant capacities. Zhang (2015) has studied the effects of solvents on the phytochemical contents. It was found that the extraction of total flavonoids significantly increased in polar solvents, which might contribute to the antioxidant activity in V. pinnata. However, in this study, ethyl acetate had higher antioxidant activity compared to the more polar methanol. This could probably mean that certain non-polar compounds, which probably dissolved better in ethyl acetate, might also contribute to the higher antioxidant activity.
In the present study, the ethyl acetate and hexane extract showed the highest and lowest radical scavenging activities amongst the extracts, respectively. Similarly, a previous study conducted by Vats (2012) on cowpea (Vigna unguiculata) also showed that ethyl acetate extract had the best DPPH radical scavenging activity. Moreover, the highest phenolic content was also found in the ethyl acetate extract, implying that most phenols were soluble in ethyl acetate. The lowest scavenging activity was in the hexane extract, which could be attributed to its low amount of phenolic content.
Closely related plant species have been shown to have similar phytochemical constituents (Diris et al., 2017). The antioxidant activities of V. pinnata’s close relatives, V. agnus-cactus, V. negundo and V. trifolia have also been determined using various solvents, and was found that the fruits and leaves of these Vitex plants were significantly capable of scavenging free radicals (Rashed, 2013; Saklani et al., 2017), signifying that Vitex plants are potential sources of promising antioxidants.
Detected zones of inhibition as shown in Table 2 indicated the presence of antibacterial activities in the extracts of V. pinnata leaves at 500 mg/mL.
The results generally showed the presence of antibacterial activities in the hexane, chloroform and ethyl acetate extracts and no antibacterial activity detected in the methanol extract. As shown in Table 2, the ethyl acetate extract could inhibit all the bacterial strains as compared to chloroform extract, which could only inhibit a total of three bacterial strains, and hexane extract, which could inhibit two strains.
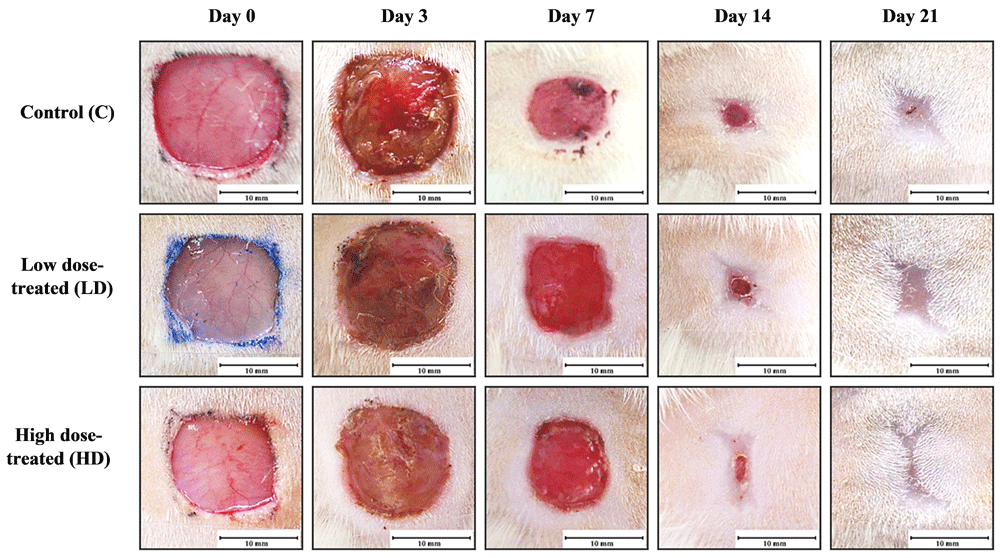
All animals were continuously observed and assessed for their general health status throughout the duration of the experiment. We did not observe any adverse effects in these animals such as infections of the excised region or any behavioral changes following the excision wounding and during the recovery period. The difference in wound area was observed from post-wounding day 7 in all experimental groups (Figure 2). The wound contraction at post-wounding day 7 was determined to be slightly higher for animals in the negative control group when compared to extract-treated groups (Figure 3). At post-wounding day 14, wound contraction in extract-treated groups were considerably higher when compared to the control group and particularly in animals receiving the higher concentration of extract (Table 3). Complete wound closure was observed in both the low dose 10% (w/w) and high dose 50% (w/w) extract treated groups at post-wounding day 21, indicating a faster rate of epithelialization compared to normal wound healing in the control group.
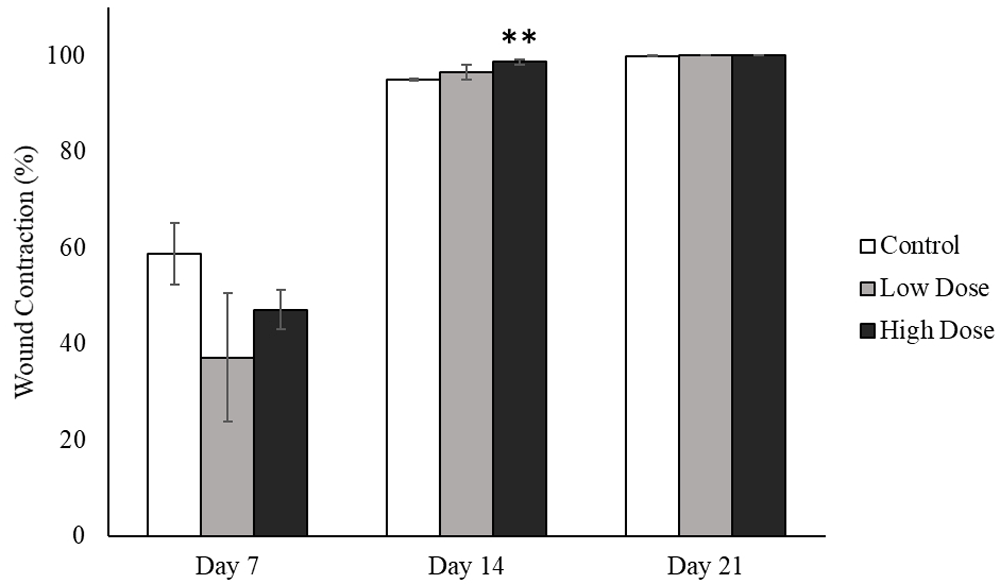
** denote significant changes of wound contraction at day 14 in comparison to control group, **p < 0.01.
| Groups | Wound contraction (%) | |||
|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | Day 21 | |
| Control | 2.4 ± 10.1 | 58.8 ± 6.4 | 95.0 ± 0.3 | 99.9 ± 0.1 |
| Low dose | -14.4 ± 19.1 | 37.1 ± 13.4 | 96.6 ± 1.6 | 100 ± 0.0 |
| High dose | -4.2 ± 8.5 | 47.1 ± 4.1 | 98.7 ± 0.6** | 100 ± 0.0 |
Values are mean ± SEM; **p < 0.01 compared to control animals on similar treatment days. The starting experiment had n=6 animals per group. One animal was sacrificed for histological analysis at day 3, day 7 and day 14. There was n=3 animals remaining at day 21 which is the final day of the experiment.
Our study demonstrated a dose dependent effect of the methanolic extract on wound healing, as evident from the wound closure observed for the 10% (w/w) vs 50% (w/w) extract treated animals. General wound morphology in post wounding day 3 shows a well-developed scab formation in all groups (Figure 2). We did not observe any adverse effects in both control and extract treated animals. At day 14, there was almost complete closure of the wound in the high dose group (Figure 2).
We observed a high density of inflammatory cells in the low dose and high dose treated tissues (Figure 4). The low dose and high dose treated animals showed epithelialization at day 7, whereas the control group showed epithelialization at day 14. Inflammatory cells become prominent, which may include neutrophils, followed by macrophages and mast cells that emigrate from nearby tissues, playing a crucial role in combating infection (Martin & Leibovich, 2005).
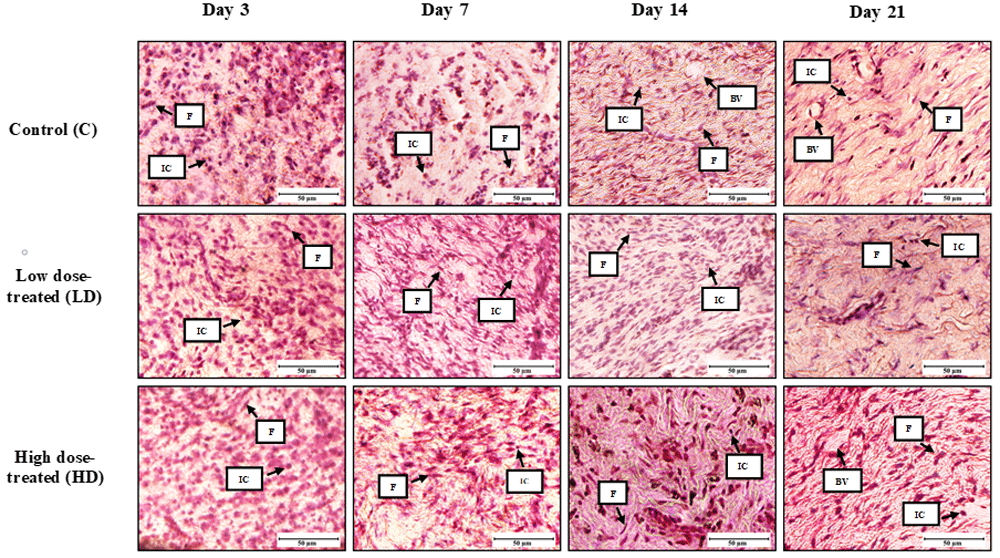
Day 3 control and treated rats: presence of inflammatory cells is evident. Collagen deposition observed in the high dose treated animals at day 14. BV: blood vessels; F: fibroblasts; IC: inflammatory cells (x400).
Based on histological observations, granulation tissue from all experimental groups displayed increased infiltration of fibroblast cells, indicative of active proliferation (Figure 4). Among all of the experimental groups, high-dose treated group showed greater wound contraction at post-wounding day 14 (Table 3). At post-wounding day 21, the wound in the high-dose treated group showed a more densely packed organization of collagen and connective tissues compared to the low dose-treated and control group.
Based on the wound contraction evaluation and histological analysis of the wound, our study has demonstrated a modest wound healing effect in the V. pinnata high-dose treated group followed by low-dose treated group as compared to control group (Figure 2). Phytochemical analysis reports have revealed that the petroleum ether, ethyl acetate, methanol and aqueous leaf extracts of V. pinnata contain varying amounts of the alkaloid, anthocyanidins, aucubins, coumarins, flavonoids, flavanols, gallic tannins, iridoids, proteins, reducing compounds, steroids, triterpenoids and glycoside compounds, of which flavonoids appeared to be present in the highest quantity in all the four different extracts (Ramesh et al., 2013). The chemical constituents of its barks have also been reported to include flavonoids (Ata et al., 2009). These phytochemicals are known to possess antioxidant, anti-inflammatory, and anti-microbial properties which are responsible for the enhanced rate of wound healing observed (Shah & Amini-Nik, 2017).
The extracts from V. pinnata leaves showed the ability to scavenge free radicals, with the ethyl acetate extract being the most potent. All extracts except for the methanol extract also exhibit species-specific antibacterial activities, with the ethyl acetate extract also being the most potent. Modest wound healing capabilities are also observed in the V. pinnata treated excision wound. Study limitations include our inability to determine the relative absorption of the plant extract into the excision wound tissue site, which may have an effect on the wound closure. Further optimization of the plant extract also needs to be carried out to elucidate factors such as toxicity and effective doses for human use.
We have focused on the effects of V. pinnata extract on an excision wound, the study could be extended to include other wound models such as burns and incision wounds in order to ascertain the plant extract’s wound healing capabilities.
Figshare: Evaluation of antioxidant, antibacterial and wound healing activities of Vitex pinnata. https://doi.org/10.6084/m9.figshare.11369973.v1 (Shafie et al., 2019)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Herbal Medicine, Plant Taxonomy
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products, Complementary and alternative medicine, vascular biology, cancer biology, non-communicable diseases.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Herbal Medicine, Plant Taxonomy
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products, Complementary and alternative medicine, vascular biology, cancer biology, non-communicable diseases.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 07 Aug 20 |
read | read |
|
Version 1 13 Mar 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (1)