Keywords
bio-electrospray, death, differentiation, human, in vitro, neural stem cells, neurone, survival
This article is included in the UCL Child Health gateway.
This article is included in the University College London collection.
bio-electrospray, death, differentiation, human, in vitro, neural stem cells, neurone, survival
Electrospraying is a very useful technique for fabricating micro- and nano-structures of different composition, texture and shape using a wide range of materials and cells. When cells are electrosprayed, the technique is known as bio-electrospray (BES). BES consists of a jet-based delivery system connected to an electric field that has the ability to generate nano-sized and cell-laden microdroplets (Jayasinghe & Townsend-Nicholson, 2006; Jayasinghe, 2011; Poncelet et al., 2012). This is due to the difference in electric potential between the charged needle and the ground electrode that forms an electric field, accelerating the charged cell suspension within the needle and forming an unstable jet. This methodology has the advantage of having the potential to achieve single-cell delivery, giving a more homogeneous cell distribution within a 3-dimensional (3D) construct, as well as being very suitable for microencapsulation (Boda et al., 2018; Jayasinghe, 2011; Poncelet et al., 2012). The configuration needed to obtain micro to nano-sized droplets and a near mono-distribution can be achieved by adjusting BES conditions (Jayasinghe et al., 2006; Jayasinghe & Townsend-Nicholson, 2006). Other strategies adopted to obtain such a high resolution require small diameter needles, resulting in shear stress to the cells and an inability to process high-density and/or viscous cell suspensions (Greig & Jayasinghe, 2008; Hall et al., 2008).
An important consideration for cell-based applications is that the high voltages and spraying action could have an adverse effect on the cells, and this may differ among cell types. Although it has been demonstrated that BES does not significantly affect a range of mammalian cells, and even small organisms, its effect have never been studied on human neural stem cells (hNSCs), the building block of the nervous system (Clarke & Jayasinghe, 2008; Geach et al., 2009; Hong et al., 2010; Jayasinghe et al., 2011; Joly et al., 2009; Tezera et al., 2017).
hNSCs either derived from the embryonic nervous system or differentiated from pluripotent stem cells provide an ideal source for modelling the human nervous system. hNSCs have the capacity to self-renew and differentiate into the major cell types of the brain, neurones and glia (oligodendrocytes and astrocytes), and hold the potential to repair damaged tissue in the central nervous system (CNS) (Bianco & Robey, 2001; Gage & Temple, 2013). This makes them invaluable for the development of 3D models for the study of normal and abnormal developmental mechanisms, neurodegenerative disorders, neural repair and high-throughput screening of putative neuroactive drugs (Breier et al., 2010; Gage & Temple, 2013; Gu et al., 2016). There is also much interest in using hNSC to develop 3D systems for transplantation into the damaged CNS (Somaa et al., 2017; Vishwakarma et al., 2014).
Given the encouraging results from a few studies on mouse neural cells and human astrocytoma (Eagles et al., 2006; Eddaoudi et al., 2010; Jayasinghe & Townsend-Nicholson, 2006; Mongkoldhumrongkul et al., 2009a), we wished to establish whether hNSCs could be bio-electrosprayed, and specifically assess whether the procedure affected their survival and ability to undergo multi-lineage differentiation. Extensive analysis of hNSC survival/death and differentiation showed that hNSCs withstand the BES procedure vey well and could successfully differentiate towards neuronal, astrocyte and oligodendrocyte lineages with no alteration in gene expression following neuronal differentiation. Together, this study demonstrates that hNSCs remain viable over prolonged periods post-treatment and are capable of withstanding the pressure and stresses of being handled as high-density cell suspensions within a needle at a high voltage.
Unless otherwise indicated, chemicals were purchased from Sigma-Aldrich (UK). Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 GlutaMAXTM (DMEM/F12), Neurobasal®-A Medium, foetal bovine serum (FBS), N-2 (100x) and B-27 (50x) supplements were from Gibco, FGF-2, EGF and PDGF-aa from Peprotech, propidium iodide (PI) from Invitrogen and Allophycocyanin (APC) Annexin V from BD Pharmingen.
Human brain embryonic tissue was provided by the Human Developmental Biology Resource (HDBR, http://www.hdbr.org/). All procedures using human tissue were carried out in accordance with the Human Tissue Act 2006 with informed consent (REC reference: 18/LO/0822) for study participation under ethical approval (NRES Committee London – Fulham, London, UK). The hNSC lines used in this study had been derived from embryonic brain tissue at Carnegie Stage (Cs)17 and Cs23, and grown on laminin, as previously described (Taylor et al., 2019; U et al., 2014; Vagaska et al., 2016). In brief, cells were seeded at a density of ~11,000 cells/cm2 and grown at 37°C in a humidified incubator with 5% CO2 in medium containing: Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) supplemented with 1% (v/v) penicillin/streptomycin, 1% (v/v) 100x N2, 2% (v/v) B27, 20 ng/ml FGF-2, 20 ng/ml EGF, 50 µg/ml, BSA fraction V, 5 µg/ml heparin and 10 µg/ml laminin.
Differentiation was induced when hNSCs had reached confluency, approximately 3 days after plating.
Neuronal differentiation. After 10 days in a medium consisting of DMEM containing Glutamax supplemented with 1% penicillin/streptomycin, 10 µM forskolin, 5 mM KCl, 2 mM valproic acid, 1 µM hydrocortisone and 5 µg/ml insulin for 10 days, cells were maintained in with Neurobasal®-A Medium supplemented with 1% L-glutamine, 1% penicillin/streptomycin and 2% B27 for 18 days (4 weeks total differentiation time). Protocol adapted from Guasti et al. (2012).
Oligodendrocyte differentiation. hNSCs were first incubated in DMEM/F12 containing 1% penicillin/streptomycin, 1% N2, 10 nM forskolin, 10 ng/ml FGF-2 and 10 ng/ml PDGF-aa for 14 days, and then in DMEM/F12 medium supplemented with 1% penicillin/streptomycin, 1% N2, 30 ng/ml tri-iodothyronine, 200 µM ascorbic acid and 10 ng/ml PDGF-aa for 7 days. PDGF-aa was then removed and cell incubated for a further 2 weeks to allow maturation (5 weeks total differentiation time).
Astrocytic differentiation. This was induced by incubating hNSCs in DMEM/F12 supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin for 2 weeks.
The BES system consisted of a high-voltage power supply (Glassman Europe Ltd., FP-30, Tadley, UK.) with a syringe pump (Harvard Apparatus) holding a needle of 1.5-mm outer diameter (0.8–0.9 mm inner diameter). The voltage was set at 10 kV and the flow rate at 250 ml/h. The procedure was carried out inside a class II biosafety cabinet to ensure sterility. hNSC suspensions with a density of ~1.3×106 cells per ml were divided into 1 experimental and 2 control groups, all run in triplicate. The experimental hNSCs were taken to the the bio-electrospray facility, which was located in a different building, and sprayed (BES group). One control group was transported to the BES facility but not sprayed (TC), and the other was left in the tissue culture laboratory (LC). All groups were replated at the same time.
The live/dead staining was performed 24 hours after BES. Hoechst 33258 and propidium iodide dissolved in phosphate buffered saline (PBS) were added to the culture medium at final concentrations of 2 μg/ml and 5 μg/ml, respectively. After a 2-hour incubation, cells were viewed and imaged using an IX71inverted microscope from Olympus equipped with a Lumen 200 metal arc lamp (Prior Scientific) and a monochrome ORCA-R2 digital camera (Hamamatsu Corp.) All images were processed with Fiji software (Java 8 version) (Schindelin et al., 2012).
Cells viability/metabolic activity was assessed 1, 3 and 6 days after BES by the MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) assay. In brief, cells were incubated for 2 hours in medium containing 10% MTT (stock solution 5 mg/ml in DMSO), after which the absorbance was measured at 595 nm with a spectrophotometer (Multiscan FC ThermoScientific).
Flow cytometry analysis was performed immediately after and 3 days after BES. Roughly 1×106 hNSCs per sample were resuspended in 500 µl of 1:100 APC-Annexin V conjugate:Annexin V binding buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCl, 5 mM MgCl2 and 1.8 mM CaCl2 adjusted with NaOH to pH 7.4). Samples were kept at room temperature (RT) in the dark for 20 minutes before adding propidium iodide (PI) to a final concentration of 5 µg/ml. Stained cells were kept on ice until loading on a BD FACSCalibur TM to carry out flow cytometry analysis. Data was analysed using Kaluza 1.3 software. As a positive control, hNSCs were treated with 10 µm thapsigargin for 24 hours to induce cell death prior to flow cytometry
Cells were fixed with 4% (w/v) paraformaldehyde (PFA) pH 7.4 for 15 minutes at RT, rinsed in PBS (phosphate buffer saline) and incubated in blocking solution (10% FBS, 3% BSA and 0.2% TritonX-100 in PBS) for 1 hour at RT. Incubation with primary and secondary antibodies at the indicated dilutions (Table 1) was overnight at 4°C, and for 1 hour at RT, respectively. The nuclear stain Hoechst 33258 (2 μg/ml) was added to the secondary antibody solution. Cells were mounted with Citifluor (Citifluor Ltd). An IX71inverted microscope (Olympus) with a monochrome ORCA-R2 digital camera (Hamamatsu Corp.) was used to acquire images. All images were processed with Fiji software (Schindelin et al., 2012).
RNA was extracted from cell pellets using RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Complementary DNA (cDNA) was prepared from 500 ng of extracted RNA using MMLV reverse transcriptase (Promega) following the manufacturers protocol. Reverse transcription reactions were performed using a PTC-100 thermal cycler (MJ Research, Inc.). The sequences of the primers and conditions used are shown in Table 2. PCR reactions were performed in a Veriti Thermal Cycler (Applied Biosciences). To exclude contamination of the reagents, no-template controls (NTC) where water instead of cDNA was included were run in each experiment. A cDNA sample from a human embryonic brain (22 weeks post conception) was used as positive controls. Amplified products were separated by gel electrophoresis using 1.5% (w/v) agarose gels in tri-acetate EDTA (TAE) buffer and 1X SYBR Safe dye (ThermoFisher Scientific). Semi-quantification of the bands was performed using Fiji software (Schindelin et al., 2012) and the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), used to normalize expression.
Each experiment was performed in biological triplicates unless stated otherwise. Statistical analysis was carried out with two-way ANOVA followed by Tukey’s multiple comparison test. Results are expressed as mean ± standard error of the mean. Differences were considered to be significant if p ≤ 0.05.
We first investigated whether hNSC viability is affected immediately after and at different times after bioelectrospraying (BES). Three groups were compared in all experiments: bio-electrosprayed hNSCs (BES) and two control groups, the BES control hNSCs (cells transported to the BES laboratory, but not sprayed; TC) and the tissue culture laboratory control hNSCs (cells not transported to the BES laboratory; LC).
At 24 hours after spraying, cells were double stained with Hoechst dye and PI to detect dead cells. No apparent difference in cell death between BES and control groups was observed by cell imaging (Figure 1A). To further assess the effect of BES on cell viability, hNSCs were labelled with PI and annexin V, a marker of apoptosis, immediately after spraying (Figure 1B) and 3 days later (Figure 1C). Cells were analysed by flow cytometry to detect early apoptotic (low PI and high APC-Annexin V), and late apoptotic/necrotic cells (high PI) as shown in Figure 1B, C. As summarized in Figure 1D, over 94% of cells were viable in all groups immediately after spraying (Day 0), and over 95% were viable at 3 days, with low levels of early apoptosis detected at both time points (1% and 0.5%, respectively). To establish whether BES affected cell behaviour over time, their metabolic activity, that reflects number of cells in the culture, was assessed by the MTT assay on day 1, 3 and 6 after BES. Metabolic activity increased over six days in all samples, although it was lower in the BES group than in the laboratory control at 1 and 3 days; however, by 6 days, no difference was observed among control and BES groups (Figure 1E). Together, these results suggest that BES does not negatively affect hNSCs viability over time.
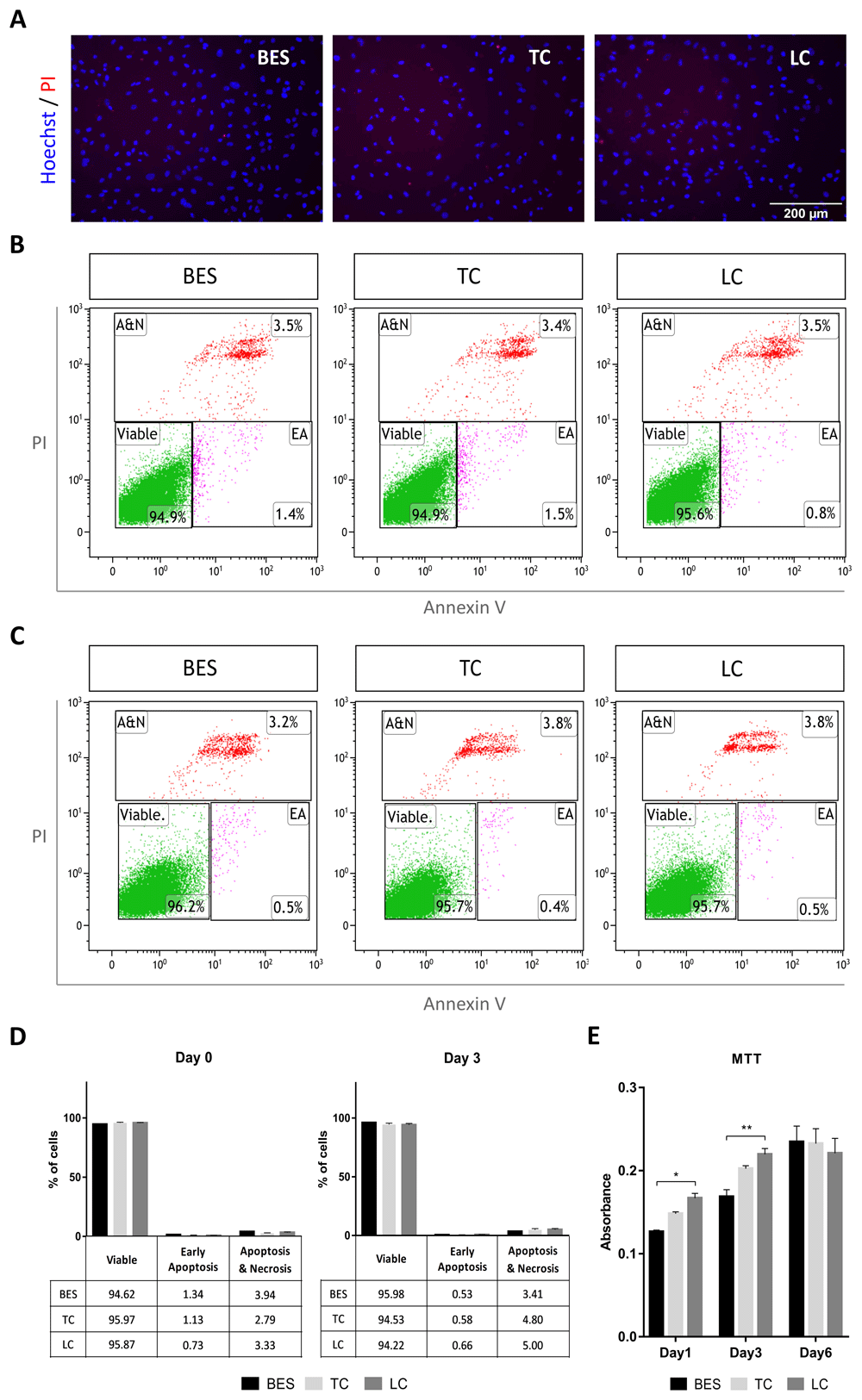
A) Staining with propidium iodide (PI, red) and Hoechst 33258 (blue) in live hNSCs (Cs 17, passage 22) 24 hours after spraying (BES) and in non-sprayed controls (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory). All pictures are at the same magnification. B–C) Analysis of Annexin V- and PI- positive cells by flow cytometry immediately after spraying (B) and 3 days after spraying (C). Representative scatter plots showing early apoptotic (EA), and apoptotic plus necrotic cell population (A&N) measured as percentages of total gated cells. D) Cell populations represented as percentages of total gated cells. No significant difference in the percentage of viable cells is observed between BES and controls (biological triplicates presented as mean ± SEM) as assessed by two way ANOVA. E) hNSC metabolic activity assessed by the MTT assay 1, 3 and 6 days after BES. Data represent mean absorbance ±SEM, n=6; * p ≤ 0.05, ** p ≤ 0.01 as assessed by two way ANOVA and Tukey’s multiple comparisons test.
To establish whether BES affected hNSCs differentiation capacity, two hNSCs lines were differentiated along the neuronal and glial lineages (astrocytes and oligodendrocytes). After 4 weeks of neuronal differentiation, processes that had started to grow at 10 days (see Extended data) had extended further in all groups, as shown by phase contrast images (Figure 2 and Extended data, Supplementary Figure S2) (Ferretti & Helenes González, 2020b). Immunofluorescence labelling for neuronal markers revealed comparable expression of the mature neuronal markers microtubule-associated protein 2 (MAP2), neurofilament 200 (NF200) and neuronal nuclear protein (NeuN) (Figure 2 and Extended data, Supplementary Figure S1 and S2) (Ferretti & Helenes González, 2020b) in the control and BES groups. In contrast, no significant expression of doublecortin (DCX), a marker of newly born and migrating neurons was detected in any group (Extended data, Supplementary Figure S1) (Ferretti & Helenes González, 2020b). These indicated that the BES process did not compromise hNSC neuronal differentiation.
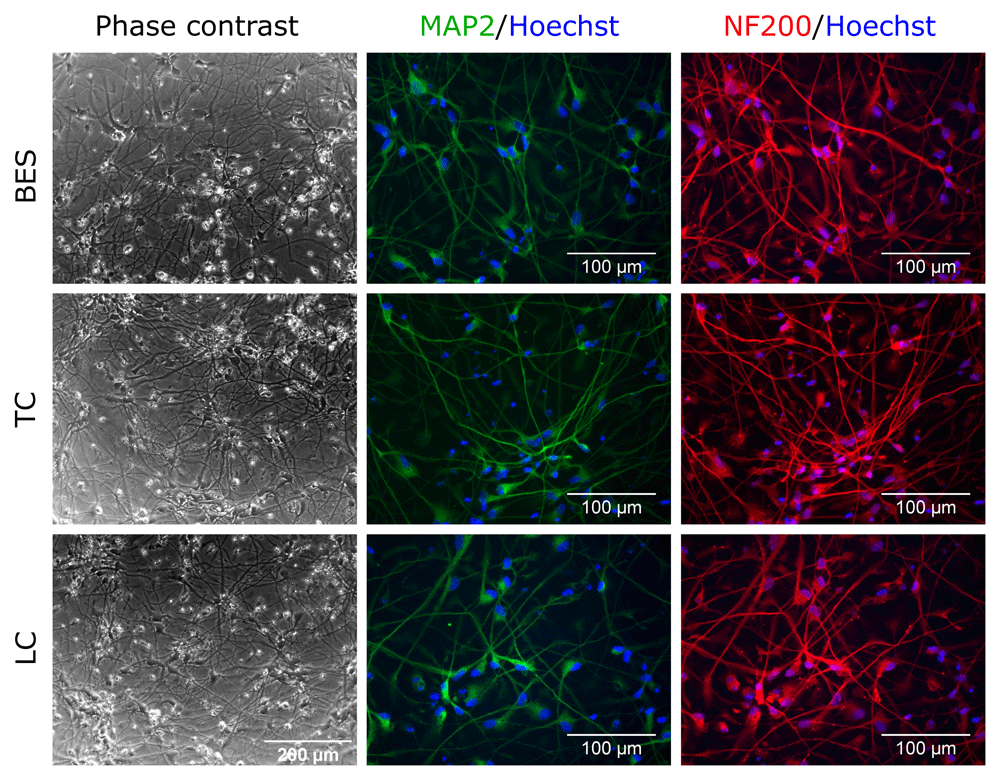
Sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 17, passage 22) differentiated for 4 weeks. Note typical neuronal morphology and neurite extension and expression of the neuronal markers, MAP2, microtubule-associated protein 2 (green), and NF200, neurofilament 200 (red). Nuclei are counterstained with Hoechst 33258 (blue). All phase contrast images are at the same magnification.
Upon induction of astrocyte differentiation, hNSC morphology rapidly changed in both control and BES groups, with cells becoming more spread and flatter than in undifferentiated controls, consistent with astrocytic differentiation (Figure 3 and Extended data, Supplementary Figure S3) (Ferretti & Helenes Gonzalez, 2020b). This was further supported by expression of vimentin and glial fibrillary acidic protein (GFAP). Together, comparable morphological appearance and glial markers expression in all groups indicates that BES does not interfere with astrocytic differentiation of hNSCs.
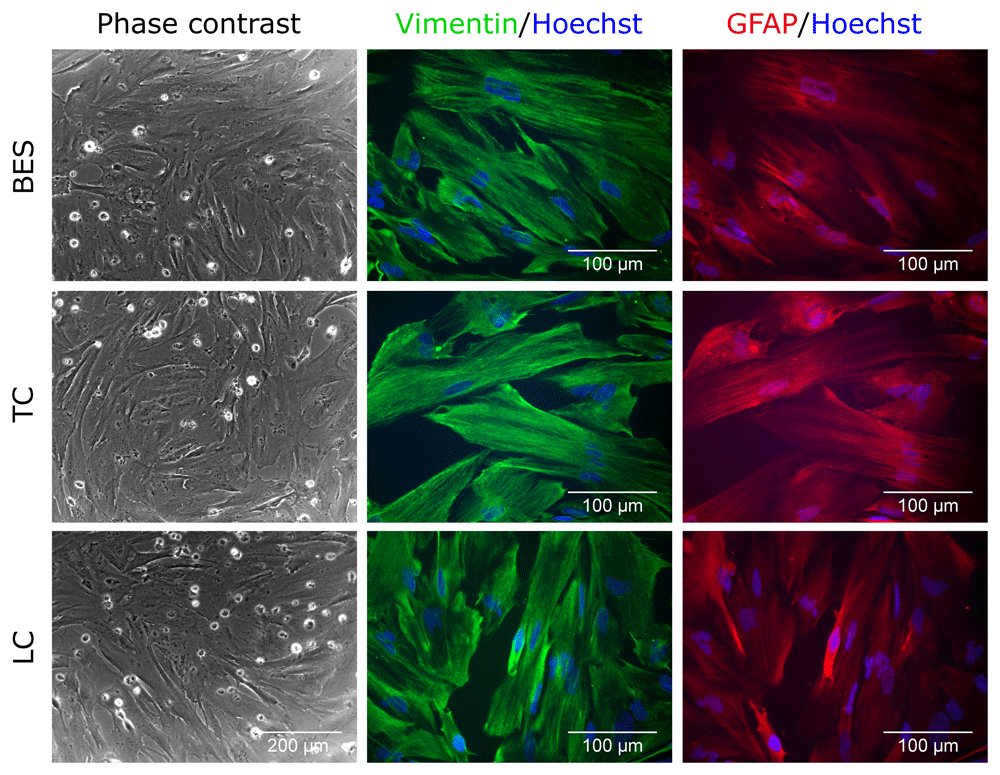
Sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 17, passage 22) differentiated for 2 weeks. Note the flatten morphology typical of astrocytes morphology and expression of astrocyte markers, Vimentin (green) and GFAP (glial fibrillary acidic protein; red). Nuclei are counterstained with Hoechst 33258 (blue). All phase contrast images are at the same magnification.
Finally, the effect of BES on oligodendrocyte differentiation was tested. At 5 weeks of differentiation after BES, both control and BES cultures had acquired a branched morphology with long processes (Figure 4 and Extended data, Supplementary Figure S4) (Ferretti & Helenes González, 2020b). Labelling of oligodendrocyte progenitor markers revealed a few cells positive for the oligodendrocyte marker, O4 (Figure 4 and Extended data, Supplementary Figure S4) (Ferretti & Helenes González, 2020b), and several positive for A2B5, that is expressed in oligodendrocyte progenitor cells (Figure 4). There was no visible difference in the expression of these markers between BES cells and their controls, indicating that the BES process does not alter the oligodendrocyte differentiation.
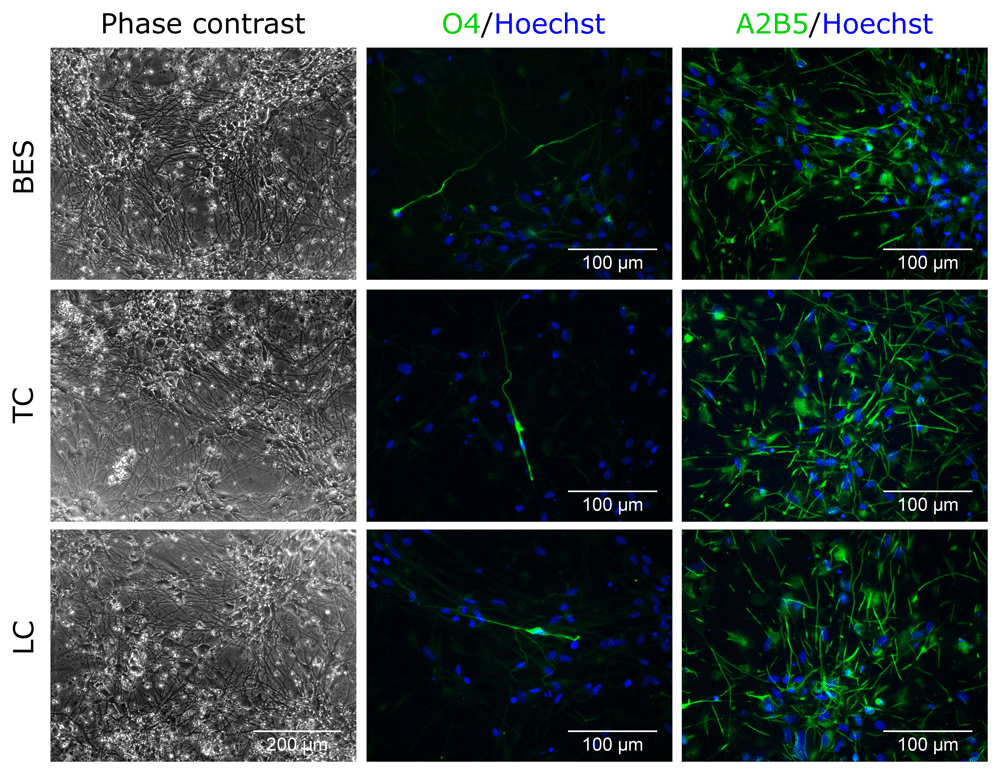
Sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 17, passage 22) differentiated for 5 weeks. Note the presence of cells with different morphologies with a few expressing the oligodendrocyte marker, O4, and a larger proportion the glial precursor marker, A2B5. Nuclei are counterstained with Hoechst 33258 (blue). All phase contrast images are the same magnification.
The effect of BES on neuronal differentiation was further investigated at the gene expression level in hNSCs after 4 weeks of neuronal differentiation. As mixed cultures are normally obtained following neuronal induction, rather than pure neuronal populations, both neuronal and glial markers were assessed to establish whether BES changed the balance of differentiation among these cell types. Transcripts for the neuronal markers, NF200, neuron-specific enolase (NSE) and MAP2, as well as the glial markers glutamate aspartate transporter (GLAST), Olig2 and GFAP were detected in all groups by RT-PCR (Figure 5A–C and Extended data, Supplementary Figure S5A–C) (Ferretti & Helenes González, 2020b). To obtain more quantitative information, gene expression was further investigated by semi-quantitative RT-PCR analysis in two hNSC lines (Figure 5D and Extended data, Supplementary Figure S5D) (Ferretti & Helenes González, 2020b). Relative expression of genes was normalised to GAPDH. No significant differences in glial marker expression between the control and BES groups was observed in either line. Also, neuronal markers were expressed at comparable levels, with the exception of NF200, which was expressed at slightly higher levels in the BES group in one of the cell lines (Extended data, Supplementary Figure S5D) (Ferretti & Helenes González, 2020b). Overall, the expression of all but one marker were unchanged, suggesting no significant effects resulted from BES.
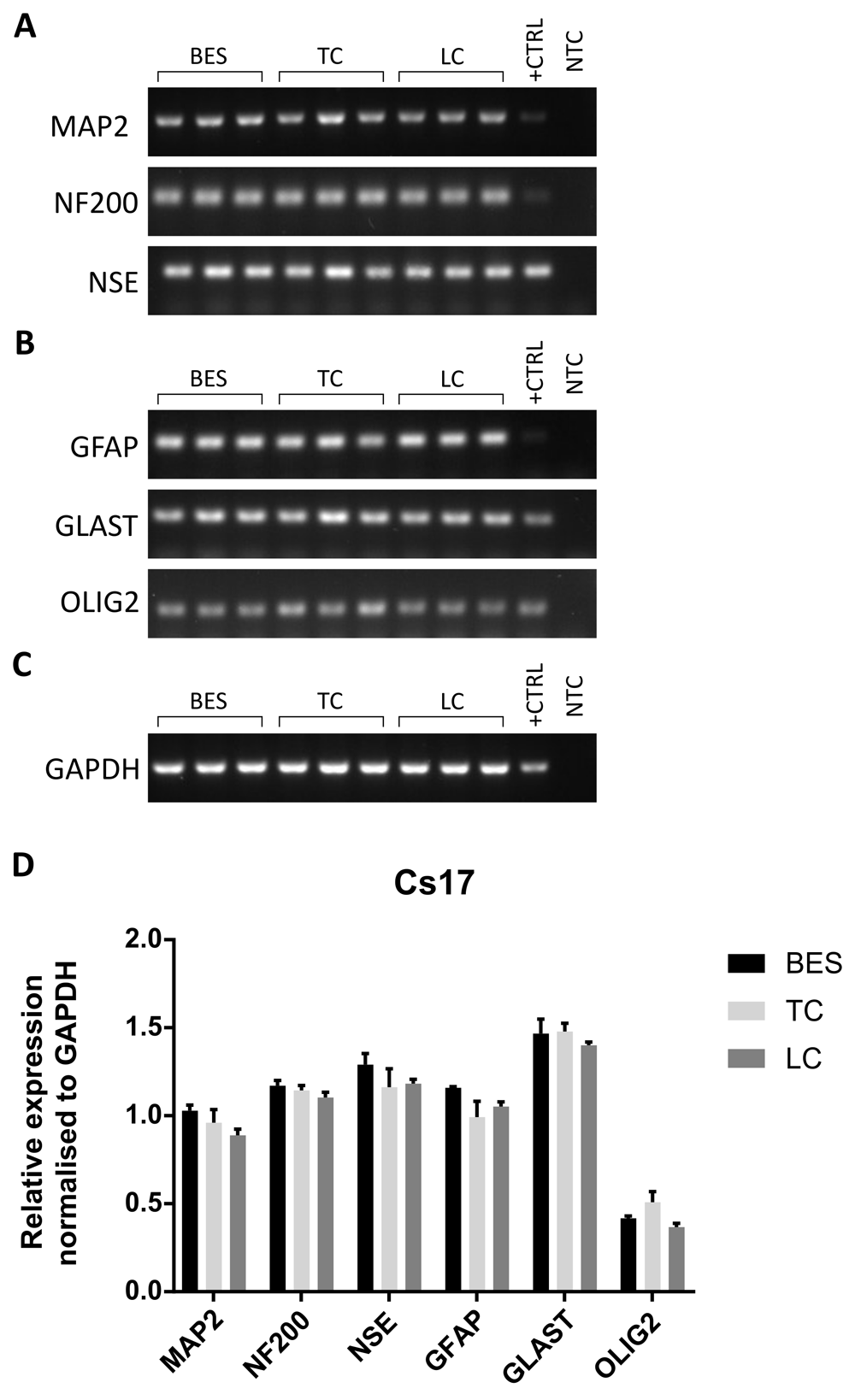
A–C. Expression of neuronal markers (A), glial markers (B), and a reference house-keeping gene, GAPDH, (C) in biological triplicates of sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 17, passage 25). MAP2: microtubule-associated protein 2; NF200: neurofilament 200; NSE: neuron-specific enolase; GFAP: glial fibrillary acidic protein; GLAST: glutamate aspartate transporter; OLIG2: oligodendrocyte transcription factor 2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase. +CTRL: human embryonic brain cDNA used as positive control; NTC: no template control using water instead of cDNA. D) Relative expression of neuronal and glial markers assessed by densitometry. Data are means ± SEM of band intensity normalised to GAPDH. No significant difference in gene expression is observed (two way ANOVA).
Only a few studies on the effect of BES on human cells have been carried out; these focussed on human mesenchymal stem cells (MSCs), either primary or hTERT immortalized, and tumour cells (Braghirolli et al., 2013; Eddaoudi et al., 2010; Mongkoldhumrongkul et al., 2009a; Ye et al., 2015). In this study, to our knowledge we show for the first time that hNSCs can withstand the BES procedure without any negative effect on their self-renewal capacity and importantly on their neuronal and glial differentiation potential. The high voltage of 10kV used here, the pressure applied by the syringe pump, the flow rate, the high-density solution in a small-bore needle (0.8–0.9 mm) and the handling of the cells in a separate laboratory had limited impact on hNSCs. It is well established that high voltages can be detrimental to cells, for example when cells are electroporated (Traitcheva & Berg, 2010). However, the fact that BES operates at high voltage but low current, in the nano-ampere range (Pakes et al., 2011; Poncelet et al., 2012), could help explain why cells do not show adverse effects when subjected to high voltages. Recently, a study assessing the effect of bioprinting on Schwann cells and myoblasts has suggested that this technique affects their viability and proliferative activity (Ning et al., 2018). Therefore, at least for some cell types, BES could provide a valuable alternative to bioprinting.
Our findings on the safety of BES on hNSCs are consistent with findings in a number of cell types and organisms previously investigated, including mesenchymal cells (Hong et al., 2010; Irvine et al., 2007; Jayasinghe et al., 2011; Ye et al., 2015), immortalised mouse neural cells and human astrocytoma cells (Eagles et al., 2006; Eddaoudi et al., 2010; Jayasinghe & Townsend-Nicholson, 2006), and nematodes (Mongkoldhumrongkul et al., 2010). In all these studies, a survival of up to 90% after BES was observed. Notably, the hNSCs displayed an even higher survival rate (>94%), suggesting that these human stem cells are more robust than most of the cells previously studied.
Another difference between previous studies and ours is the length of time we monitored the cells for. Here, not only we measured metabolic activity up to 6 days, but also monitored the cells over weeks in the differentiation experiments, where undifferentiated controls were run in parallel. The use of two controls also showed that an initial small decrease in metabolic activity on BES samples was partly due to the transfer of the samples to the BES laboratory, and that full recovery had occurred by day 6. Viability of rabbit bone marrow-derived MSCs after BES was lower than that of hNSCs, with a metabolic/proliferation rate significantly lower than in controls even at a lower bio-electrospraying voltage than that used in our study (Sahoo et al., 2010). Together, the combination of cell death and survival assays and long-term monitoring used here provides clear evidence that BES does not affect hNSCs viability either immediately or over time.
Our tri-lineage differentiation study has clearly shown no changes in hNSC differentiation potential after BES. As from previous reports, hNSC induction of neuronal differentiation resulted in a heterogeneous population of cells, and this was comparable across groups as shown by mRNA expression (Glaser et al., 2007; Gu et al., 2016; Sun et al., 2008). Astrocytic differentiation resulted in a more homogeneous population and all cells expressed vimentin and GFAP, though at different extents. The low number of cells positive for the oligodendrocyte marker O4 upon induction of oligodendrocyte differentiation is comparable in control and BES groups, and consistent with the long time required for human oligodendrocyte maturation. Indeed, a marker of less mature cells, A2B5, was expressed in a much higher proportion of cells.
This again supports the view that hNSCs are very resistant to external stimuli. This may be a property of human stem cells or of neural stem cells, or both, and extensive investigation will be required to compare stem cell types across species to clarify this issue. Rabbit bone marrow-derived MSCs have been shown to maintain differentiation potential along three mesenchymal lineages after BES at a lower voltage than the one used here (Sahoo et al., 2010). By contrast, human MSCs derived from human deciduous tooth pulp appear to better withstand BES even at higher voltage (15 kV), as well as maintain tri-lineage differentiation potential (Braghirolli et al., 2013; Braghirolli et al., 2015). Also human adipose-derived MSCs (ADSCs) survived and differentiated efficiently after BES (Ye et al., 2015).
Analysis of cell viability, tri-lineage differentiation capacity and gene expression demonstrated that the BES process does not adversely affect hNSCs either in the short or long term. Notably, it highlighted the robustness of these human stem cells. In conclusion, this study shows that BES is a suitable tool for the direct handling of hNSCs. Therefore, it may provide a suitable technology for deposition of hNSCs to specific locations in damaged nervous system in vivo or within suitable scaffolds for neural tissue engineering. Furthermore, this approach could be developed to generate well-controlled human neural 3D models for studying neural development or disease and responses to putative novel therapeutic interventions.
Harvard Dataverse: Bio-electrosprayed human neural stem cells are viable and maintain their differentiation potential- Underlying data of main figures. https://doi.org/10.7910/DVN/CAASEG (Ferretti & Helenes González, 2020a).
This project contains the raw uncropped images used to produce each figure, in addition to flow cytometry, cell viability and RT-PCR output data.
Harvard Dataverse: Bio-electrosprayed human neural stem cells are viable and maintain their differentiation potential- Underlying data of supplementary figures. https://doi.org/10.7910/DVN/CLGEWR (Ferretti, 2020).
This project contains the raw uncropped images used to produce each of the supplementary figures (see Extended data), in addition to RT-PCR output data for Supplementary Figure S5D.
Harvard Dataverse: Bio-electrosprayed human neural stem cells are viable and maintain their differentiation potential- Extended data. https://doi.org/10.7910/DVN/M8ZFNR (Ferretti & Helenes González, 2020b).
This project contains the file ‘Supplementary figures.pdf’, which contains the following extended data:
Figure S1 Expression of neuronal markers in hNSCs after 4 weeks of differentiation. (A–B) Neuronal nuclear protein (NeuN) and doublecortin (DCX) in sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) in two hNSC lines, Cs 17, passage 22 (A) and Cs23, passage 20 (B). Nuclei are counterstained with Hoechst 33258 (blue).
Figure S2. Neuronal differentiation of hNSCs after bio-electrospray assessed by phase contrast imaging and double-labelling for neuronal markers. Sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 23, passage 20) differentiated for 4 weeks. Note typical neuronal morphology and neurite extension and expression of the neuronal markers, MAP2, microtubule-associated protein 2 (green), and NF200, neurofilament 200 (red). Nuclei are counterstained with Hoechst 33258 (blue). All phase contrast images are at the same magnification.
Figure S3. Astrocyte differentiation of hNSCs after bio-electrospray assessed by phase contrast imaging and double-labelling for astrocyte markers. Sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 23, passage 20) differentiated for 2 weeks. Note the flatten morphology typical of astrocytes morphology and expression of astrocyte markers, Vimentin (green) and GFAP (glial fibrillary acidic protein; red). Nuclei are counterstained with Hoechst 33258 (blue). All phase contrast images are at the same magnification.
Figure S4. Oligodendrocyte differentiation of hNSC assessed after bio-electrospray by phase contrast imaging and immunostaining. Sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 23, passage 20) differentiated for 5 weeks. Note the presence of cells with different morphologies with a few expressing the oligodendrocyte marker, O4, and a larger proportion the glial precursor marker, A2B5. Nuclei are counterstained with Hoechst 33258 (blue). All phase contrast images are the same magnification.
Figure S5. Expression of neural markers in hNSCs neuronally differentiated for 4 weeks after bio-electrospray assessed by RT-PCR. (A-C) Expression of neuronal markers (A), glial markers (B), and a reference house-keeping gene (C) in biological triplicates of sprayed (BES) and control (TC: taken to the BES laboratory but non-sprayed; LC: not moved from the tissue culture laboratory) hNSCs (Cs 23, passage 22). MAP2: microtubule-associated protein 2; NF200: neurofilament 200; NSE: neuron-specific enolase; GFAP: glial fibrillary acidic protein; GLAST: glutamate aspartate transporter; OLIG2: oligodendrocyte transcription factor 2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase. +CTRL: human embryonic brain cDNA used as positive control (22 weeks post conception); NTC: no template control using water instead of cDNA. (D) Relative expression of neuronal and glial markers assessed by densitometry. Data are means ± SEM of band intensity normalised to GAPDH. Increased NF200 expression (* p 0.05) is observed in the BES group (two way ANOVA with Tukey’s multiple comparisons test).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
CHG designed and performed experiments, analysed data, obtained funding and wrote the manuscript; SNJ advised and provided training on bio-electrospray and reviewed the manuscript; PF planned research, obtained funding, and wrote the manuscript.
We wish to thank Dr Dale Moulding at the ICH Microscopy Facility for his advice on image acquisition and Dr Ayad Eddaoudi for help with flow cytometry data acquisition.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tissue engineering and regenerative medicine - area of stem cell and nanotechnology (electrospinning mainly)
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Electrospray, Biomaterials, Tissue Regeneration
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tissue engineering & Synthetic Biology (but my reservations, which are purely about more methodological details being needed in places, do not depend on any deep expertise).
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 31 Jul 20 |
read | read | |
|
Version 1 17 Apr 20 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)