Keywords
Ebola virus, antiparasitic drugs, antiviral activity
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Ebola Virus collection.
Ebola virus, antiparasitic drugs, antiviral activity
Since its discovery in 1976, Ebola virus has been responsible for numerous outbreaks, with case fatalities varying from 20% to 90%1,2. From 2014 to 2016, West Africa was devastated with the largest Ebola outbreak in recorded history with six countries affected: Nigeria, Senegal, Guinea, Liberia, Mali and Sierra Leone. With no effective therapies or licensed vaccines available, little could be done to treat patients and prevent the spread of the virus3. Over a period of two years, Ebola virus was responsible for 28,646 reported infections, with 11,323 deaths1,3.
Since the outbreak, a vaccine for Ebola virus (rVSV-EBOV) has been developed by the National Microbiological Laboratory in Winnipeg, Manitoba, Canada, and has been shown to be highly protective4. Undoubtedly, the rVSV-EBOV vaccine is to be a powerful defense against future Ebola outbreaks. However, it is unlikely to be sufficient to stop future outbreaks. As the natural host of Ebola virus remains unknown, eradication of Ebola reservoir populations is impossible5. Because of this, future spillover events are an inevitability6. Additionally, the unstable political environment of many affected African countries further complicates the effective administration of vaccines; as seen in the Democratic Republic of Congo during the current Ebola outbreak2,7. These factors emphasize the need for acute infection therapies.
Previous publications have focused on potential drugs predicted to have efficacy against Ebola virus disease (EVD) and included drugs already approved and widely used that could perhaps be repurposed as treatments for EVD8,9. Through a virtual screening process, Veljkovic et al.8 identified 267 US Food and Drug Administration (FDA)-approved drugs with possible inhibitory effects against Ebola virus. Among these were the antimalarial quinoline drugs amodiaquine and chloroquine. Chloroquine has been shown to be protective against Ebola virus disease in an in vivo mouse model9. Additionally, amodiaquine has been identified as having inhibitory effects in a pseudo-type entry assay of Ebola virus9. Furthermore, retrospective analysis of relative risks for patients from the 2014 to 2016 West Africa outbreak identified amodiaquine in combination with artesunate as having therapeutic effects. Data have suggested that patients infected with Ebola virus that were prescribed artesunate-amodiaquine had a significantly reduced risk of death compared to those that were prescribed artemether-lumefantrine, the latter treatment not being statistically significantly different from no treatment10. This suggests that the quinoline derivative drug class may have therapeutic use in the treatment of EVD.
In this article, the efficacy of 36 novel quinolines (Table 1) derivatives and previously approved quinoline compounds (amodiaquine and chloroquine) were examined for their ability to inhibit Ebola virus replication. Our results support previous reports that suggested that amodiaquine and chloroquine could be potential treatments for EVD. In addition, we identified additional, novel, quinolines that could be candidates for further study of their potential for inhibition of Ebola virus infection.
The column on the left indicates the percent inhibition of trVLP replication using 10 µM, and the column on the right indicates the drug toxicity as assessed using MTT viability.
Inhibition: HEK 293T cells were either transfected with replication machinery plasmids VP30, VP35, NP and Tim-1 (-L) or transfected with all replication machinery plasmids and Tim-1, allowing trVLP entry and replication (+L). Cells were treated with a 10µM final dose of each quinoline derivative, two hours pre-infection. Cells were infected with 100µL of viral stock diluted in 200µL of DMEM with 5% FBS. Cells were lysed, and luciferase activity was assessed 72 hours post-infection. Data represents three biological replicates. Data background corrected and displayed as a percentage of the positive control. Error shown is standard error of the mean. Viability: Drugs were added at a final dose of 10µM, 0.1% DMS. 26 hours later, media was replaced with 100µL of 0.5 mg/mL MTT in media without phenol red. After incubating for two hours at 37°C, 100 μL 10% SDS was added. MTT was fully dissolved at 37°C, then absorbance was read at 570nm. Wells containing no cells served as blanks. Cell viability was determined as percent absorbance of treatment to 0.1% DMSO control. Data shown represents four biological replicates. Data background corrected and displayed as a percentage of the no treatment control. Error shown is the standard error of the mean.
Chloroquine diphosphate (Chl) was purchased from Abcam (catalog #ab142116) and amodiaquine dihydrochloride dihydrate (Amo) was purchased from Sigma-Aldrich (catalog #A2799) Artesunate (Art) was provided by the BioCIS research group at University Paris-Saclay, France. Novel quinoline derivatives: 2PentQ11,12, 2OHQ12, 2NH2Q13, 2COOHQ14, 2CNQ15,16, 2QQ17, 2Qi1518, 2Q16OH18, 2QPOH19, 2PQSel11, DD120, DD221, DD3 (Desmaële unpublished; see Extended data)22, DD4, (Desmaële unpublished; see Extended data)22, DD5 (Desmaële unpublished; see Extended data)22, MBN11120, MBN13220, MBN9120, KHD29123, KHD28823, MBN115 (Desmaële unpublished; see Extended data)22, MD823 (Desmaële unpublished; see Extended data)22, FZ4924, DD624, DD7 (unpublished; see Extended data)22, DD8 (Desmaële unpublished; see Extended data)22, FZ14224, TOF41125, TOF40125, MD20 (Desmaële unpublished; see Extended data)22, MBN8720, MBN14020, FS48 (Desmaële unpublished; see Extended data)22, AS65 (Desmaële unpublished; see Extended data)22, XF90617 and BS46011 were synthesized at University Paris-Saclay, France. Among them, 2PQsel and 2OHQ have exhibited in vitro and in vivo activities against the parasite Leishmania spp.26.
HEK-293T (American Type Culture Collection; ATCC, Rockville, USA) were grown in polystyrene coated, 75 cm2 flasks (Sarstedt) in 15 mL of Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS at 5% CO2 atmosphere and 37°C. Cells were harvested by washing using phosphate-buffered saline, followed by incubation with 2mL of trypsin/EDTA for 5 minutes in 5% CO2 atmosphere at 37°C.
To evaluate the efficacy of quinoline compounds of possible inhibition of Ebola virus, a replication competent mini-genome system developed by Hoenen et al.27 was adopted. Transcription and replication competent viral-like particles (trVLP) are used to model the Ebola virus lifecycle. Within the trVLPs resides, a mini-genome containing structural viral proteins (VP24, VP40, and GP1,2) with the addition of a luciferase reporter gene. To facilitate viral replication, target cells (HEK-293T) are transfected with expression plasmids containing the remaining Ebola replication machinery proteins (VP30, VP35, NP, and L) 24 hours prior to infection. This model represents the most comprehensive lifecycle model available while still being able to be conducted in a biosafety level 2 facility27–31.
As described by Hoenen et al.27, producer HEK-293T cells were seeded at 2.5 million cells/mL into 10cm Petri dishes in 10mL of DMEM containing 10% FBS at 5% CO2 atmosphere and 37°C. After 24 hours, cells were transfected with expression plasmids for the Ebola tetracistronic minigenome, virus replication machinery proteins, and bacteriophage T7 polymerase. The plasmids used have been previously described elsewhere29 and were: tetracistronic minigenome; p4cis-vRNA-Rluc, T7 polymerase; pCAGGS-T7, viral protein 30; pCAGGS-VP30, viral protein 35;pCAGGS-VP35, viral NP; pCAGGS-NP, viral polymerase L; pCAGGS-L. All transfections were carried out using the calcium phosphate method (CalPhos Mammalian Transfection Kit, Clontech Laboratories; catalog #631312). Plasmids are described in Hoenen et al.27. At 24 hours post-transfection, media was replaced with 15 mL of DMEM with 5% FBS. trVLPs containing supernatants were collected after 72 hours and stored at -80°C.
To assess the efficacy of compounds for inhibition of viral replication in vitro, 40,000 293T cells, quantified using a hemocytometer, were seeded in 400µL of DMEM containing 10% FBS. After 24 hours, seeding cells were transfected with replication machinery expression plasmids as well as the cellular Ebola virus attachment factor Tim-1 (plasmid pCAGGS-Tim1) using the CalPhos Mammalian Transfection Kit (Clontech Laboratories; catalog #631312)27. 24 hours following transfection, media was removed, followed by the addition of 300µL DMEM with 5% FBS. To this, 6µL of 100X drug stock prepared in 10% DMSO w/w was added. Two hours post-treatment, 100µL of viral stock diluted in 200µL of DMEM with 5% FBS, warmed to 37°C, was added to cells. The following day, media was replaced with 800µL of DMEM with 5% FBS. 72 hours post-infection, media was removed and cells were lysed in 200µL of 1x Renila Luciferase Assay Lysis Buffer (Renilla Luciferase Assay System, Promega; catalog #E2820). Luciferase activity in 20µL of lysate was measured using a Luminoskan Ascent microplate luminometer (Thermo Electron) after the addition of 100µL of Luciferase Assay Substrate diluted in Assay Buffer (Renilla Luciferase Assay System, Promega; catalog #E2820).
Cells were seeded into 96-well plates at a density to mimic day two (post-transfection) stage (30% confluent). Drugs were added to the final dose, 0.1% DMSO, and 26 hours later, media was replaced with 100 μL 0.5 mg/mL 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) in media without phenol red. After incubating for two hours at 37°C, 100μL 10% SDS was added. MTT was fully dissolved at 37°C, then absorbance was read at 570nm using a Biotech Epoch 2 spectrophotometer. Wells containing no cells served as blanks. Cell viability was determined as a percentage of absorbance of the background-corrected 0.1% DMSO treatment control.
Means were compared using the two-tailed, paired Student’s t-test (assuming equal variances) using Microsoft Excel, version 2016. A p-value ≤0.05 was considered significant. Error bars for Table 1 infection data are the range for n=2.
To assess the ability of quinolines to inhibit Ebola virus infection, we employed an established mini-genome model of Ebola replication, trVLP, allowing us to work under biosafety containment level 2 (CL 2) conditions27–31.
In our first series of tests, the ability of amodiaquine and chloroquine to inhibit in vitro infection of Ebola virus replication was tested (Figure 1). Following transfection with the required replication machinery and attachment receptor expression plasmids, HEK-293T cells were treated with the drugs at a concentration of 10 µM two hours pre-infection. Luciferase activity was measured 72 hours post-infection22. Both amodiaquine and chloroquine demonstrated significant reductions in luciferase activity, indicating inhibited viral transcription and replication32. Following these findings, dose-response experiments for amodiaquine and chloroquine were conducted. Inhibition activity was assessed at concentrations ranging from 10 µM to 0.31 µM (Figure 2A and 2B). Amodiaquine exhibited a half maximal inhibitory concentration (IC50) of 1.45 µM, while chloroquine exhibited an IC50 of 3.95 µM. Cellular toxicity for both amodiaquine and chloroquine was measured using an MTT viability assay (Figure 2A and 2B) to quantify drug toxicity along the concentration response22.
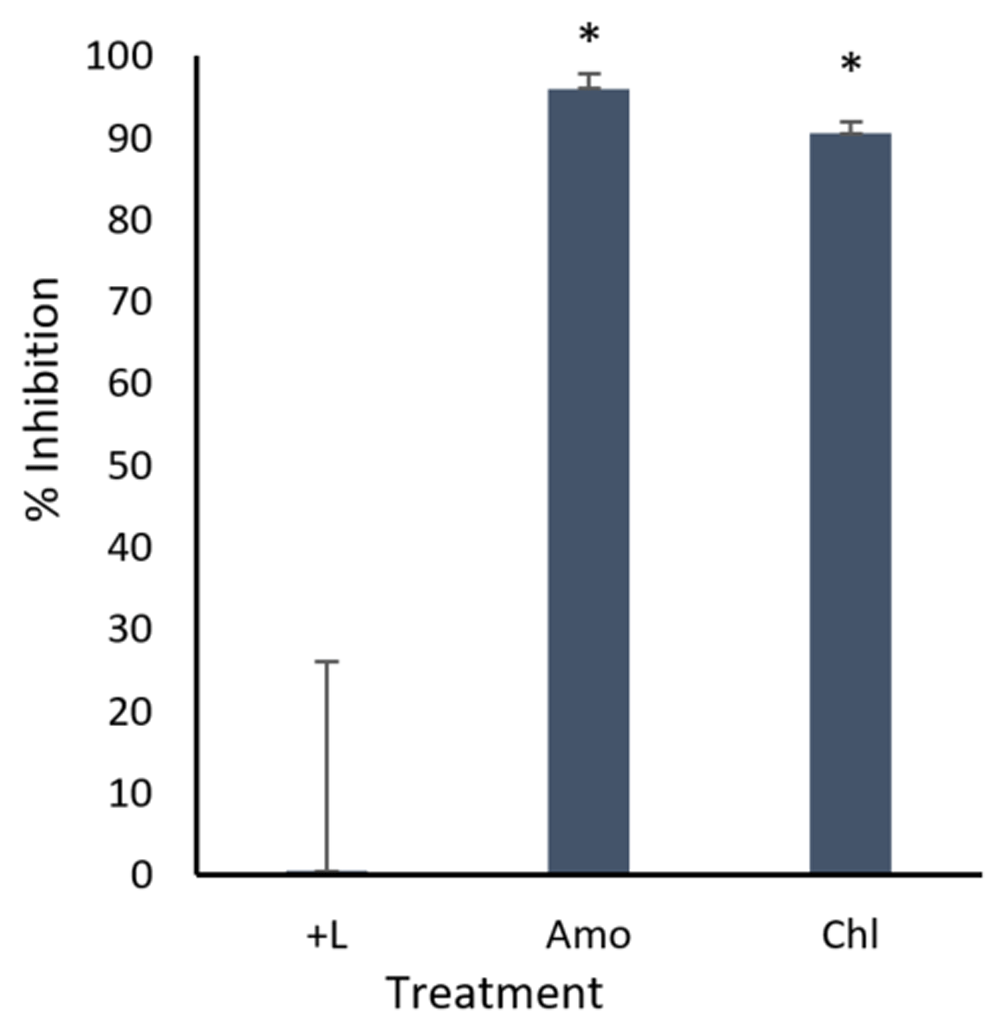
293T cells were either transfected with replication machinery plasmids VP30, VP35, NP and Tim-1 (-L) or transfected with all replication machinery plasmids and Tim-1, allowing trVLP entry and replication (+L). Cells were treated with either 10µM amodiaquine or chloroquine two hours pre-infection or received no treatment. Cells were infected with 100µL of viral stock diluted in 200µL of DMEM with 5% FBS. Cells were lysed, and luciferase activity assessed 72 hours post-infection. Data shown represents four biological replicates. Data background corrected and displayed as a percentage of the positive control. Error bars shown are the standard error of the mean. Results from drug treatments were statistically compared with the (+) control group. * Denotes a p-value <0.05.

Circular markers, solid line: 293T cells were either transfected with replication machinery plasmids VP30, VP35, NP and Tim-1 (-L) or transfected with all replication machinery plasmids and Tim-1, allowing trVLP entry and replication (+L). Cells were treated with either amodiaquine, chloroquine or 2NH2Q at concentrations of 10, 5, 2.5, 1.25, 0.625, or 0.31µM, two hours pre-infection. Cells were infected with 100µL of viral stock diluted in 200µL of DMEM with 5% FBS. Cells were lysed, and luciferase activity assessed 72 hours post-infection. Data shown represents four biological replicates. Data background corrected and displayed as a percentage of the positive control. Error bars shown are the standard error of the mean. Square markers, dashed line: 293T cells were seeded to mimic day two (post-transfection) stage (30% confluency). Drugs were added at a final dose of 0.47, 0.94, 1.88, 3.75, 7.5, 15, or 30 µM, 0.1% DMS. 26 hours later, media was replaced with 100µL of 0.5 mg/mL MTT in media without phenol red. After incubating for two hours at 37°C, 100 μL 10% SDS was added. MTT was fully dissolved at 37°C then absorbance was read at 570nm. Wells containing no cells served as blanks. Cell viability was determined as a percentage of the absorbance of treatment to 0.1% DMSO control. Data shown represents three biological replicates. Data background corrected and displayed as a percentage of the no treatment control. Error bars shown are the standard error of the mean.
To rapidly assess the inhibitory ability of the 36 novel quinoline derivatives (Table 1), drugs were screened at a concentration of 10 µM as described above, with drug treatments occurring two hours pre-infection. From these tests, 2OHQ12, 2NH2Q13, 2QQ17, 2Qi1518, MD823, TOF411, DD2, DD1, and MBN91 were identified as having inhibitory activity in our in vitro assay (Table 1). However, due to drug toxicity or availability of synthesized drug, only 2NH2Q was pursued for further study. A dose-response for 2NH2Q was conducted, with inhibition being assessed between 10 µM and 0.31 µM (Figure 2C). Results indicated the IC50 of 2NH2Q to be 4.66 µM, slightly higher than either amodiaquine or chloroquine.
As outbreaks of EVD continue to occur, treatment and preventative strategies are urgently needed.In August 2018, the Ministry of Health of the Democratic Republic of the Congo declared a new outbreak of Ebola virus disease in North Kivu Province. As of January 19, 2019, there have been 636 confirmed cases of EVD and 370 confirmed deaths2,7. Previously, it was suggested that anti-malarial drugs may be effective for the treatment of EVD8–10. In the report, we have provided supporting evidence that antiparasitic drugs can inhibit Ebola virus replication in vitro, which could be a breakthrough in fighting EVD if proven by additional studies or clinical trials.
Quinoline is a heterocyclic aromatic organic compound and is the base structure for many small molecule drugs. Quinoline-based drugs are widely used for the treatment of malarial infections. As their production is cheap, they are easy to synthesize with high yields, and are chemically stable while being readily accessible, quinolines offer many advantages for the treatment of infectious disease in tropical climates, such as the climates found in Africa8,27.
Our study aimed to test anti-malarial compounds against an Ebola replication model using the previously published trVLP in vitro assay, which has been shown to accurately reflect CL4 studies using intact infectious Ebola virus29. We find amodiaquine to be effective at inhibiting Ebola viral replication. Amodiaquine is a useful anti-malarial agent in regions where Ebola virus outbreaks are possible, and this drug has been suggested to help with EVD outbreaks10. We also show using our assay that other antiparasitic malarial drugs can inhibit Ebola virus replication with low cellular toxicity. These include chloroquine, 3/35 novel synthetic quinolines, one termed 2NH2Q, with high efficacy in inhibiting Ebola virus replication and low toxicity, and 4/35 showing moderate efficacy in inhibiting Ebola virus replication while being relatively non-toxic (Table 1).
Quinolines, such as amodiaquine or chloroquine, have been used in regions at risk for EVD to prevent malaria and some have stated that use of these compounds is correlated with reduced death from EVD, suggesting that quinolines could be useful as both an anti-malarial and EVD treatment/prevention drug.
In summary, 38 compounds selected for their antiparasitic activity were evaluated against Ebola virus and their cytotoxicity was also determined. Some of them had antileishmanial activity, such as 2PQsel13 and others exhibited antiplasmodial activity, such as chloroquine, amodiaquine and BS46033. The most interesting compounds emerging from viral inhibition and cytotoxicity assays were chloroquine and amodiaquine, with the advantage of chloroquine having no toxicity at 10 µM. From the quinoline series, the most promising compounds were 2Qi15 and 2NH2Q, both of them being 2-substituted quinolines. When studying structure-activity relationships, the presence of an amino group at the extremity of the propylene chain (compound 2NH2Q) allowed a better cell viability (81%) than a hydroxyl group (compound 2OHQ, 31%), despite their similar capacity for viral inhibition (89%). Replacement by a carboxylic group (compound 2COOHQ) strongly reduced the antiviral inhibition but maintained good cell viability. The other substitutions performed on the quinoline scaffold were not really satisfactory in regard to their biological activity.
Our results support the hypothesis that antiparasitic quinolines active against malaria and leishmaniasis could be efficacious as a treatment for EVD. Further studies are necessary to confirm the utility of quinolines as anti- Ebola virus therapeutics, including consideration of these drugs when designing future Ebola drug trials in Africa.
Harvard Dataverse: Inhibition of in vitro Ebola infection by anti-parasite quinoline derivatives. https://doi.org/10.7910/DVN/ONGV6I22
This project contains the following underlying data:
Harvard Dataverse: Inhibition of in vitro Ebola infection by anti-parasite quinoline derivatives. https://doi.org/10.7910/DVN/ONGV6I22
This project contains the following extended data:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We wish to thank Mrs. B. Seon-Meniel (BioCIS) for her help in the preparations of the drugs.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medicinal Chemistry, heterocyclic compounds
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioinformatics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Apr 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
From the 36 quinoline-type compounds investigated, 3 (2NH2Q, NQi15 and DD2) demonstrated a significant inhibitory activity for Ebola trVLP replication and low toxicity. From these, theauthors have proposed2NH2Q, with an IC50of 4.66 µM, asa leading candidate for further studies. In the discussion section the authors comment on SAR for some of the compounds, namely a selection of compounds that exhibit different terminal functionalities (amino, hydroxy or carboxyl) on the substituent in position 2, claiming that a terminal amine, as in 2NH2Q, is better for activity and toxicity. It is expected that further research to unravel the mode of action will shed light on substituent effects, while showing further directions for lead optimization.
The quest for drugs to fight EVD is of upmost urgency and, from the relevant results now disclosed, 2-substituted quinolines appear as a valid chemotype. This work deserves publication.
From the 36 quinoline-type compounds investigated, 3 (2NH2Q, NQi15 and DD2) demonstrated a significant inhibitory activity for Ebola trVLP replication and low toxicity. From these, theauthors have proposed2NH2Q, with an IC50of 4.66 µM, asa leading candidate for further studies. In the discussion section the authors comment on SAR for some of the compounds, namely a selection of compounds that exhibit different terminal functionalities (amino, hydroxy or carboxyl) on the substituent in position 2, claiming that a terminal amine, as in 2NH2Q, is better for activity and toxicity. It is expected that further research to unravel the mode of action will shed light on substituent effects, while showing further directions for lead optimization.
The quest for drugs to fight EVD is of upmost urgency and, from the relevant results now disclosed, 2-substituted quinolines appear as a valid chemotype. This work deserves publication.