Keywords
transcutaneous bilirubin, preterm neonates, predict, hyperbilirubinemia
transcutaneous bilirubin, preterm neonates, predict, hyperbilirubinemia
Hyperbilirubinemia is a common condition occurring in neonatal periods1, with a prevalence of around 60% in term neonates and 80% in preterm neonates. Preterm neonates have greater risk of severe hyperbilirubinemia, which can lead to encephalopathy2. This condition is preventable if early detection and prompt treatment can be arranged and managed properly1,3,4.
Visual assessment is not reliable especially in the first 24–48 hours, since only 80% of jaundiced babies can be recognized visually if the bilirubin level reaches > 6 mg/dL5–8. High bilirubin levels can be dangerous, since preterm neonates have a greater risk of low bilirubin kernicterus2.
Total serum bilirubin (TSB) measurement remains the gold standard for diagnosing hyperbilirubinemia. The drawbacks of this procedure, however, are that it is painful, causes stress to the neonates, has a greater risk of infection, and needs a couple of hours to get the results9–11.
Trancutaneous bilirubinometry (TcB) is a non-invasive procedure to identify hyperbilirubinemia. A number of studies have been conducted to validate TcB to assess whether it can be used safely. These found that TcB has good correlations with TSB. The use of TcB can also reduce the need for blooding sampling by 41–73%10,12,13.
Due to the burdens of an occurrence of hyperbilirubinemia, its early detection and prediction are crucial. TSB or TcB is recommended to predict neonatal hyperbilirubinemia for neonates with >35 weeks of gestation13–15. Some studies using TcB to predict hyperbilirubinemia have already been conducted, but all of them recruited only late preterm and term neonates5,16. For preterm neonates, one study was already conducted using TSB measurement at the age of 6 until 24 hours to predict hyperbilirubinemia in the following hours or days4. As far as the researchers know, there has been no previous study using TcB to predict hyperbilirubinemia for preterm neonates. Therefore, the aim of this study was to use TcB to predict hyperbilirubinemia in preterm neonates to prevent complications since visual assessment is no longer reliable.
This was a cohort study conducted in the Neonatal Intensive Care Unit (NICU) at Dr Soetomo General Hospital for 5 months (September 2018–January 2019). This study was approved by Dr. Soetomo General Hospital Surabaya Ethics Committee (No. 0586/KEPK/Ix/2018). An informed consent was signed by parents after they understood the information for consent. Study size retrieved in this research used purposive sampling with inclusion and exclusion criteria (a flow diagram is available as Extended data)17,18 during the research period. The sample size that we retrieved was estimated by applying Hulley et al.19 formulation of which confidence interval was at 95%, coefficient correlation at 0.84 and standard deviation of 1.84. Therefore, we applied minimum sample of 20 samples for each group, classified by infants’ body weight in certain ranges. While staying in accord with the minimum sample size, we expanded our samples up to 45 infants for each group with total sample of 90 infants. Yet, we had excluded four data due to missing TSB measurement.
Race and thickness of melanin layer of skin tissue were taken into account as confounding variables, and as variables able to modify and differ the outcomes of others. Therefore, to control for study bias, the subjects addressed for this study were those subjects which had similar ethnic backgrounds, which were Malay Mongoloid.
The inclusion criteria were: 1) born at ≤35 weeks of gestational age and with a birth weight of <2000 g, and 2) parental consent by signing a form. The exclusion criteria were: 1) being diagnosed as hyperbilirubinemia at the age of 12 hours, 2) having any major congenital anomaly, 3) being discharged from hospital at an age of less than 3 days. Neonates who received phototherapy before the observation was done, missed TSB, or voluntarily resigned from this study were excluded from the study. The subjects recruited were divided into two groups, neonates with birth weights of 1001–1500 g (Group I) and 1501–2000 g (Group II).
The bilirubin level of each neonate was measured on the sternum by TcB (Dräger® Jaundice Meter 105) at the age of 12 hours, 24 hours, and 72 hours with ±3 hours tolerance (the TcB measurement could be taken within 3 hours before/after the exact time). The TSB measurement was taken for each neonate at the age of 3 days or if the TcB bilirubin level was ≥5.76 mg/dl for group I and TcB ≥8.76 mg/dl for group II and it had to be taken within 6 hours before/after the TcB measurement. The TSB measurement also had to be taken if the TcB measurement showed abnormal results. Hyperbilirubinemia was defined as TSB ≥7 mg/dl for preterm neonates with birth weights of 1000–1500 g and TSB >10 mg/dl for preterm neonates with birth weights of 1501–2000 g.
The data was analysis by Microsoft Office Excel, IBM SPSS Statistics Version 21. We performed receiver operating characteristic (ROC) curve analysis to determined cut off point of TcB level to predict hyperbilirubinemia at the age of 48 and 72 hours. We calculated the specificity, sensitivity, positive predicted value (PPV), negative predicted value (NPV), and likelihood ratio.
There were 90 preterm neonates recruited for this study, 40 of whom weighed 1000–1500 grams (Group I) and 50 of whom weighed 1501–2000 grams (Group II). Only 38 neonates of group I and 48 neonates of group II were observed until the end of the study. Four neonates were excluded from the study due to missing TSB results.
Maternal and neonatal characteristics are shown in Table 1. For group I, the mean gestational age of group I was 32.29 ± 1.84 weeks, with a mean birth weight of 1273.68 ± 177.34 grams. Meanwhile for group II the mean gestational age was 33.69 ± 1.26 weeks and a mean birth weight of 1792.70 ± 145.86 grams. Based on risk factors of ABO-incompatibility, one subject of group I who suffered hyperbilirubinemia at the age of 48 hours. Meanwhile, two subjects in group II suffered hyperbilirubinemia at the age of 48 hours and another two subjects at the age of 72 hours at the end of observation, the maximum bilirubin level was 15.2 mg/dl for group I and 16.33 mg/dL for group II. Most neonates of group I (44.7%) suffered hyperbilirubinemia at the age of 48 hours, while most neonates of group II (45.8%) at the age of 72 hours (Figure 1). We found the TSB mean in group I at the age of 24, 48, and 72 hours to be 7.9 mg/dL, 9.16 mg/dL, and 9.3 mg/dL respectively, and 11.01 mg/dL, 10.23 mg/dL, and 11.04 mg/dL respectively, in group II.
A ROC curve was constructed to determine a hyperbilirubinemia threshold based on the data collected. We found that the AUC (area under curve) of the TcB bilirubin level at the age of 12 hours to predict hyperbilirubinemia at the age of 48 hours for group I was 0.804 (p 0.002) with a cut-off point of 2.35 mg/dL (sensitivity 79.20% and specificity 71.40%). For the TcB bilirubin level at the age of 24 hours to predict hyperbilirubinemia at the age of 48 hours, we found an AUC 0.771 (p 0.06), with a cut-off point of 4.50mg/dl (sensitivity 87.50% and specificity 64.26%) (Figure 2a, Figure 2b, Table 2).
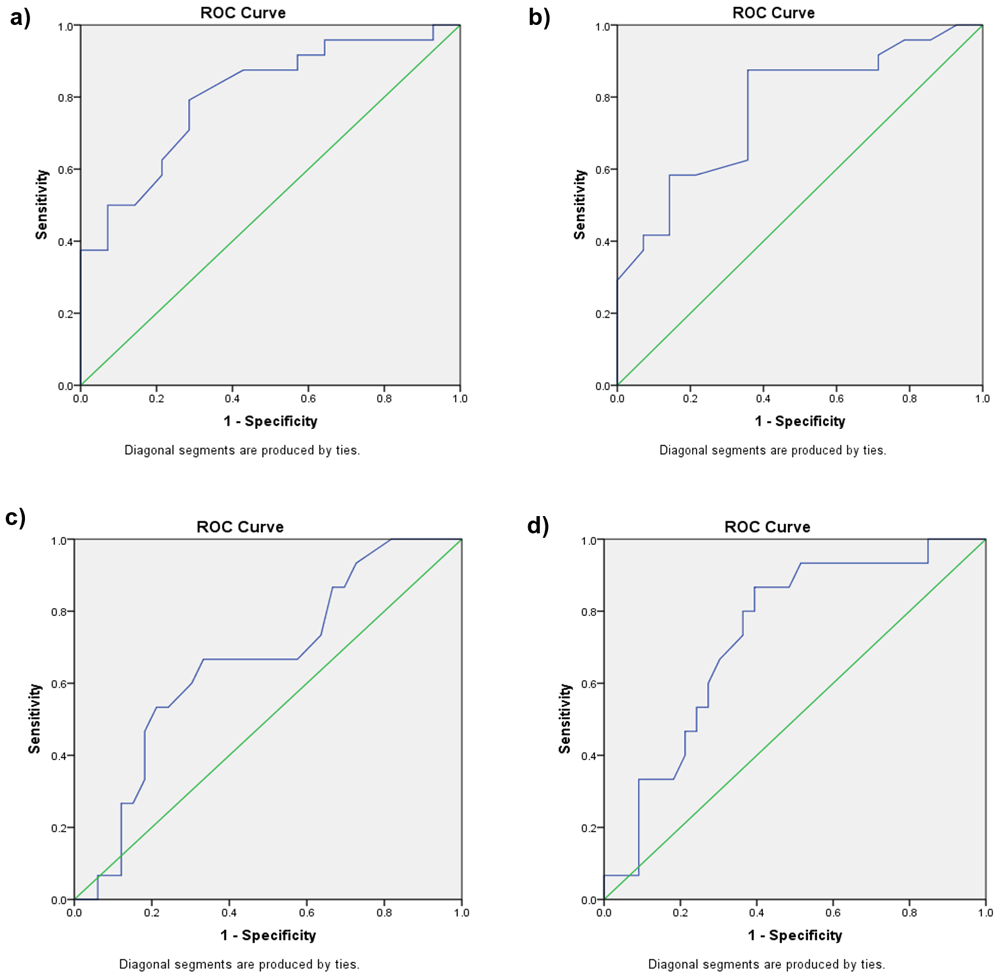
(a) Receiver operating characteristic (ROC) curve for TcB at the age of 12 hours to predict hyperbilirubinemia at the age of 48 hours for group I. (b) ROC curve for TcB at the age of 24 hours to predict hyperbilirubinemia at the age of 48 hours for group I. (c) ROC curve for TcB at the age of 12 hours to predict hyperbilirubinemia at the age of 48 hours for group II. (d) ROC curve for TcB at the age of 24 hours to predict hyperbilirubinemia at the age of 48 hours for group II.
We found the AUC of TcB bilirubin levels at the age of 12 hours to predict hyperbilirubinemia at the age of 48 hours for group II was 0.658 (p = 0.083), with a cut-off point of 3.05 mg/dL (sensitivity 66.7% and specificity 66.7%). The AUC of TcB bilirubin level at the age of 24 hours was 0.732 (p = 0.011), with a cut-off point of 5.80 mg/dL (sensitivity 80% and specificity 63.6%). (Figure 2c, Figure 2d and Table 3).
The TcB bilirubin level of group I at the age of 12 hours, 24 hours, and 48 hours to predict hyperbilirubinemia at the age of 72 hours showed a very weak AUC, which were 0.243 (p = 0.386); 0.297 (p = 0.494); 0.500 (p = 1.000) respectively, therefore no cut-off point could be determined.
The TcB bilirubin level of group II at the age of 12 hours to predict hyperbilirubinemia at the age of 72 hours showed a weak AUC (0.499; p = 0.991) with a cut-off point of 2.65 mg/dL (sensitivity 60% and specificity 46%). At the age of 24 hours, we found TcB AUC 0.751 (p = 0.008), with a cut-off point of 5.15 mg/dL (sensitivity 74.3% and specificity 76.9%). Meanwhile, at the age of 48 hours the TcB AUC was 0.731 (p = 0.015), with a cut-off point 8.65 mg/dL (sensitivity 67.6% and specificity 61%) (Figure 3a–c and Table 4).
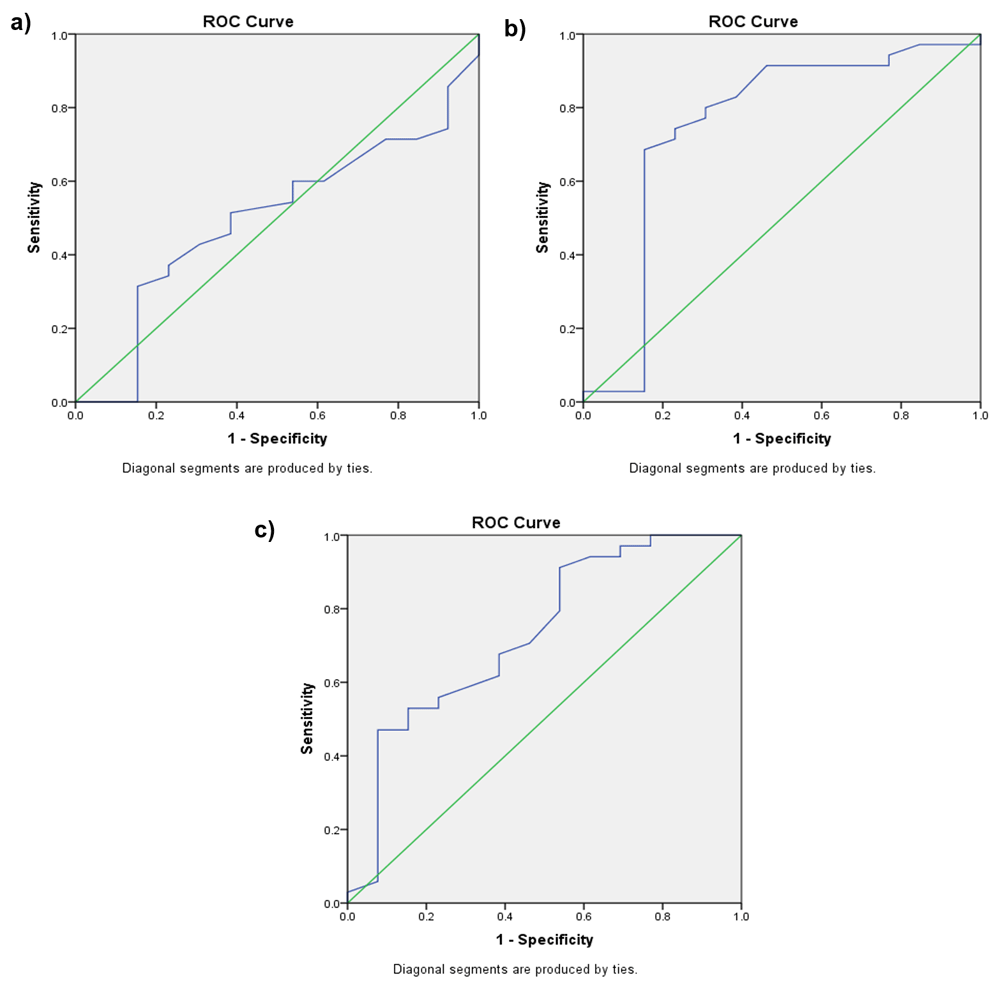
(a) Receiver operating characteristic (ROC) curve for TcB at the age of 12 hours to predict hyperbilirubinemia at the age of 72 hours for group II. (b) ROC curve for TcB at the age of 24 hours to predict hyperbilirubinemia at the age of 72 hours for group II. (c) ROC curve for TcB at the age of 48 hours to predict hyperbilirubinemia at the age of 72 hours for group II.
This study has determined a TcB cut-off value of 4.5 mg/dL at the age of 24 hours in group I (1000–1500 grams) and 5.8 mg/dL in group II (1501–2000 grams) as predictive of hyperbilirubinemia at the age of 48 hours. To predict hyperbilirubinemia at the age of 72 hours, this study could not determine any TcB cut-off value for group I (1000–1500 gram) as a result of very weak correlation. The TcB cut-off value of 5.15 mg/dL at the age of 24 hours was determined as the best predictor for hyperbilirubinemia at the age of 72 hours in group II (1501–2000 grams). This cut-off level was established with a sensitivity value ranging from 74.3% to 87.5% at 24 hours after birth. Similar studies have already been conducted, but those studies recruited only late preterm neonates. Lavanya et al. found that TcB values measured in the first 24–48 hours of life can predict hyperbilirubinemia at an age of more than 48 hours20. Bansal et al. determined that TcB values of >4.6 mg/dl at the age of 12–24 hours (sensitivity 83.09%; specificity 87.37%; PPV 90.4% and NPV 78.3%) and >7.4 mg/dl at the age of 24–48 hours (sensitivity 93.55%; specificity 82.11%; PPV 81.69% and NPV 95.35%) are predictors for hyperbilirubinemia in the first 48 hours of life5. Other studies conducted used TSB values to predict hyperbilirubinemia in the following days. Mayer recruited preterm neonates weighing 1000–1500 grams and determined a capillary TSB value of 3.55 mg/dl at the age of 12 hours as the best predictor of significant hyperbilirubinemia (sensitivity 94.4%, PPV 98.1%, and NPV 40%)4.
Most neonates recruited in this study suffered hyperbilirubinemia before the age of 72 hours old. This study also showed that smaller babies suffered peak incidence earlier (at 48 hours) than larger babies (at 72 hours). Hyperbilirubinemia is more prevalent in preterm neonates4,5,20) and is usually more severe and has longer duration compared to that in term neonates4. This is caused by increased bilirubin production, decreased bilirubin excretion, increased enterohepatic circulation, lower albumin level and a weak albumin-bilirubin bond11,21. Early detection of hyperbilirubinemia will decrease its mortality and morbidity. The need for reliable methods to predict hyperbilirubinemia is important. The use of a noninvasive procedure, like TcB, in the first 6–24 hours of life is recommended as a marker of bilirubin production22 and it can decrease the need for blood sampling23.
In group I at the ages of 24, 48, and 72 hours, we found TSB mean values of 7.9, 9.16, and 9.3 mg/dL, respectively; in group II, these values were 11.01, 10.23, and 11.04 mg/dL, respectively. This is similar to previous study which found that TSB mean values in the first 5 days of the lives of preterm neonates were 10–12 mg/dL24. However, this study found there were preterm neonates who reached a TSB value >15 mg/dL in the first 72 hours. However, it is possible for preterm neonates to have high bilirubin levels in the first days of life, which can lead to hyperbilirubinemia complications if not recognized and treated properly. The previous study conducted by Bhutani et al. also indicated that neonates who suffer from hyperbilirubinemia in the following days had higher percentiles on the first day of life25. Therefore, the American Academy of Pediatrics (AAP) recommends routine checks of TSB or TcB along with risk factor assessments in the first days of life14.
To the knowledge of the researchers, this was the first study conducted to predict hyperbilirubinemia in preterm neonates weighing 1000–2000 grams using TcB. One limitation of the study was that it could not determine TcB cut-off values to predict hyperbilirubinemia at the age of 72 hours for preterm neonates weighing 1000–1500 grams due to a lack of subjects able to complete the study, since most had already developed significant hyperbilirubinemia by this time. This, it is hoped that future similar studies will be able to recruit larger populations.
TcB values in the early days of life can be used as a predictor of hyperbilirubinemia in the following days for preterm neonates. It is possible for preterm neonates to have high bilirubin levels in the first few days of their lives. Therefore, daily measurement of TcB is important for early identification of hyperbilirubinemia, especially in order to prevent complications in certain more vulnerable preterm neonates. Close monitoring should be arranged for those who have TcB values higher than the cut-off values.
Figshare: Datasheet TcB and TSB - Group 1. https://doi.org/10.6084/m9.figshare.11948490.v126.
This project contains data gathered for neonates in group 1 (those 1000–1500 grams).
Figshare: TcB Level and TSB-MTA - Group 2. https://doi.org/10.6084/m9.figshare.11948529.v127.
This project contains data gathered for neonates in group 2 (those 1500–2000 grams).
Figshare: Supplemental File - Flow Chart Study of TcB and TSB. https://doi.org/10.6084/m9.figshare.12017586.v118.
This project contains a study flow diagram.
Figshare: STROBE checklist for ‘Transcutaneous bilirubin level to predict hyperbilirubinemia in preterm neonates’. https://doi.org/10.6084/m9.figshare.11991672.v217.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank neonatal unit at Dr. Soetomo Academic Teaching Hospital members who also gave contributions within the study progress, as follows:
1. Siti Annisa Dewi Rani, MD and Muhammad Pradhika Mapindra, MD as research assistants whom are employed in our Neonatal Unit for data editing
2. Spencer Lemaich who contributed to proofread the manuscript in English;
3. Head of each Neonatal Unit ward: Mrs. Pamiani, Mrs. Wahyu, and Mrs. Peni for supporting our study and coordinating each of their nursing team to collaborate with us;
4. All our colleagues in Neonatal Unit at Dr. Soetomo Academic Teaching Hospital.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Global Health, Neonatal Hyperbilirubinemia, Pediatric Critical Care
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bilirubin, Jaundice
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 08 Oct 20 |
||
|
Version 2 (revision) 07 Sep 20 |
read | read |
|
Version 1 28 Apr 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)