Keywords
Ustekinumab, Pediatric Crohn's disease, anti-TNF-refractory Crohn's disease, Inflammatory bowel disease, Therapeutic drug monitoring, Clinical response
Ustekinumab, Pediatric Crohn's disease, anti-TNF-refractory Crohn's disease, Inflammatory bowel disease, Therapeutic drug monitoring, Clinical response
Together, ulcerative colitis and Crohn’s disease (CD) make up inflammatory bowel disease (IBD), an autoimmune-mediated process of unclear etiology. The global incidence of pediatric IBD has been rising rapidly, with the highest incidence of CD being in Europe at 23/100000 person years and North America at 15.2/100000 person years1–3. Earlier onset of IBD is associated with higher impact on growth and development, more aggressive disease course, and increased need for immunomodulators4. Anti-tumor necrosis factors (anti-TNFs) form the forefront of management of patients with CD who do not respond to steroids and immunomodulatory medications5.
Among pediatric patients with CD who are started on anti-TNF treatments, about 10–25% do not respond to it (primary non-responders)6. Of those who initially respond, loss of response and adverse effects limit duration of therapy. At 1, 3, and 5 years after therapy initiation, the probability of patients remaining on infliximab is only 0.87, 0.74, and 0.67, respectively (secondary non-responders)7. Thus, there is a significant need for novel therapies for management of CD.
Among the newer biologics approved for treatment of CD is ustekinumab, a human immunoglobulin G1 kappa monoclonal antibody that binds with high affinity to the p40 subunit of human interleukin (IL)-12 and IL23. Ustekinumab prevents IL12 and IL23 bioactivity by preventing their interaction with their cell surface receptor protein IL12Rb1. Through this mechanism of action, ustekinumab effectively neutralizes IL12 (Th1)- and IL23 (Th17)-mediated cellular responses. It has recently been approved for the treatment of moderate to severe active CD in adults8. However, data on usage of ustekinumab in management of pediatric Crohn’s disease is limited to small case series9–11. Here we describe our experience on using ustekinumab for management of TNF-refractory pediatric CD.
We performed a retrospective chart review on 10 pediatric CD patients who failed anti-TNF therapy and were treated with ustekinumab between January 2016 and November 2018.
This study was approved by the Institutional Review Board (IRB) of Dallas Children’s Hospital (study #25338). Request for waiver of patient/guardian consent for this study was approved by the IRB.
We collected baseline demographic data, disease phenotype based on Paris Classification12, disease related complications, previous treatment history, and reason for changing therapy.
To assess clinical response to ustekinumab, we calculated the Abbreviated Pediatric Crohn’s Disease Activity Index (abbrPCDAI) prior to starting therapy, 2–3 months after therapy initiation, and at the last office visit before conclusion of the study13. When no office visits were available immediately prior to treatment initiation, telephone and email encounters were used to assess patients’ clinical symptoms to calculate abbrPCDAI. Where possible we also calculated the Simple Endoscopic Score for Crohn’s Disease (SES-CD) before and after treatment initiation14. Body Mass Index (BMI) before and after treatment was collected.
Laboratory measurements, which include hematocrit, C-reactive protein (CRP), and albumin, were also collected before and after treatment initiation. We also looked at the trough ustekinumab levels where available in relation to dose and response to therapy.
Patients are categorized as anti-TNF primary non-responders if there’s no clinical response during therapy induction, and secondary non-responders if there’s loss of response during maintenance phase15. Based on abbrPCDAI, clinical response is defined as ≥15 points reduction, and clinical remission is defined as <10. We define sustained clinical remission as abbrPCDAI of <10 with no subsequent elevation in AbbrPCDAI as of the last visit. Disease severity is categorized as follows: severe <25; moderate 16-25; mild <16. We use the following SES-CD cutoff to define disease severity: remission 0-2; mild 3-6; moderate 7-15; and severe >1616. Endoscopic response is defined as ≥50% decrease in SES-CD score compared to baseline17. Based on previous studies we used a target ustekinumab trough level of >4.5 µg/mL18.
Patients’ age at initial diagnosis ranged from 2 to 14 years (median age of 9.5 years). Age at initiation of ustekinumab ranged from 9 to 19 years (median age of 14.5 years). Duration of disease ranged from 3 to 14 years (median duration of 6.5 years). Table 1 summarizes patients’ demographic, disease phenotype at diagnosis, extraintestinal manifestations, disease related surgeries, treatment history, and reasons for changing therapy to ustekinumab. Of note, all 10 patients in our cohort were refractory to anti-TNF therapy.
EIM- extra intestinal manifestations.
Table 2 summarizes the ustekinumab induction and maintenance dose used in these patients. For induction, the dosing varied among patients with 7 out of 10 receiving induction doses per current recommendations: patients with weight <55kg received either 6mg/kg or 260mg, 55-85kg received 390mg, and >85kg received 520mg. For the remaining three patients, one received 2 doses of 45mg every 4 weeks (Q4) for induction; the second patient was induced on two separate times, 1.5 years apart, he received 90mg the first induction and 390mg for the second; the third patient was induced with 90mg Q4 for 3 doses.
| Patient | Weight (kg) | Induction (mg) | Maintenance (mg) | Initial interval (weeks) | Trough level (ug/ml) | Final maintenance dose (mg) | Final intervals (weeks) | Last trough level (µg/mL) | Therapy duration (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 32.2 | 45x2 | 45 | 8 | NA | 90 | 8 | NA | 142 |
| 2 | 103.8 | 520 | 90 | 8 | undetectable | 90 | 4 | NA | 59 |
| 3 | 46.6 | 260 | 90 | 8 | 3.6 | 90 | 4 | 8 | 63 |
| 4 | 38.9 | 240 | 90 | 6 | 0.8 | 90 | 4 | 7.1 | 71 |
| 5 | 29.4 | 180 | 45x1, then 90 | 6 | 0.1 | 90 | 6 | >10 | 73 |
| 6 | 73.2 | 90 | 90 | 4 | NA | 90 | 4 | NA | 4 |
| 6* | 86.7 | 390 | 90 | 4 | 10 | 90 | 8 | NA | 16 |
| 7 | 39.1 | 90x3 Q4 | 90 | 8 | 1.1 | 90 | 6 | NA | 143 |
| 8 | 50.8 | 260 | 90 | 8 | NA | 90 | 8 | NA | 55 |
| 9 | 73.2 | 390 | 90 | 8 | NA | 90 | 8 | NA | 36 |
| 10 | 70.2 | 390 | 90 | 8 | NA | 90 | 8 | NA | 25 |
For maintenance, 6 of the 10 patients received 90mg every 8 weeks (Q8), while 3 patients received 90mg every 6 weeks (Q6), and 1 patient received 45mg Q8. One patient was on ustekinumab on two separate occasions, the first time 90mg Q4 for two doses, and the second time 90mg Q4, then Q8 once therapeutic level and remission were achieved. Subsequently, two patients required frequency escalation to Q4 weeks, and one of them went back to Q6 after she went into remission. One patient required escalation to 45mg Q5, and then back to 90mg Q8 when disease was controlled. Another patient was maintained on Q8 for 32 months before he relapsed and required increase in dosing frequency to Q6. Maintenance frequency was titrated based on clinical response and/or ustekinumab trough level. Duration of therapy ranges from 4–135 weeks (median 61 weeks).
Of the seven patients who received augmented maintenance doses, all seven showed clinical response, as shown in Figure 1 (patients 1-7), and all but one patient achieved sustained clinical remission as assessed by abbrPCDAI. One patient achieved remission after 5 months of specific carbohydrate diet (SCD) and ustekinumab at Q8 maintenance. He remained in remission for the next 17 months, then developed fatigue and bloody stool when diet was liberalized. His symptoms did not respond to reintroduction of SCD. However, he went into clinical remission when ustekinumab maintenance interval was changed to Q6 weeks.
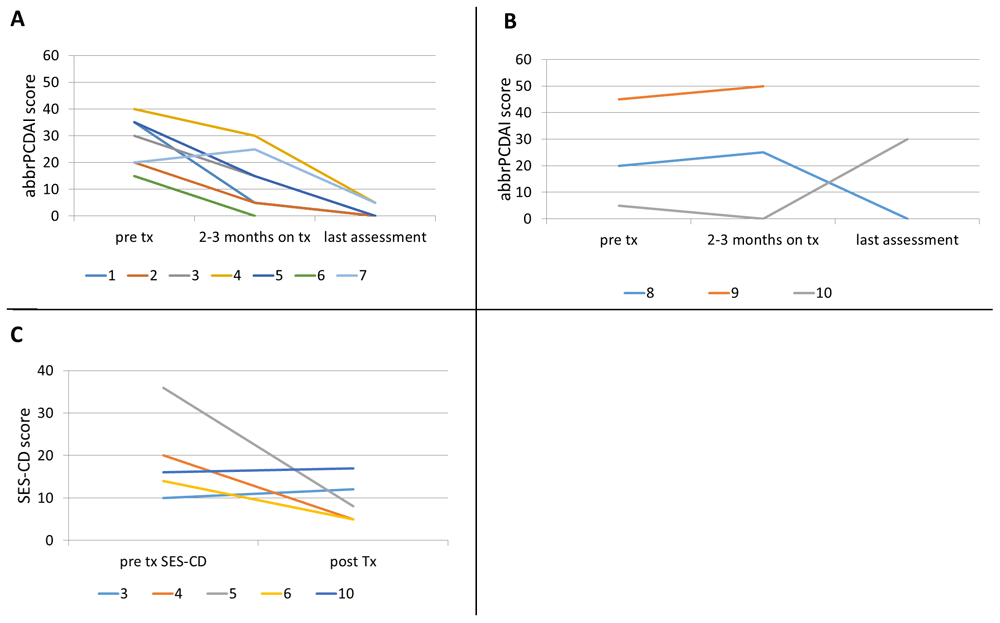
Abbreviated-pediatric Crohn’s disease activity index in patients with (A) augmented ustekinumab dosing and (B) Q8 dosing; (C) simple endoscopic score-Crohn’s disease (SES-CD) pre and post-ustekinumab initiation.
Only 4 out of 10 patients had endoscopy before and after ustekinumab treatment. Of these, three patients showed endoscopic response and one showed worsening of SES-CD score (Figure 1C). While no patient showed mucosal remission, mucosal inflammation did improve from severe to mild, severe to moderate, and moderate to mild in three patients.
Laboratory indices also improved in 6 out of 7 patients (Figure 2). The most significant and consistent improvements were seen in CRP and albumin (Figure 2C–F). BMI improved significantly in patients with pre-treatment BMI below the 2nd percentile, and either decreased or showed small numerical improvement in patients with pre-treatment BMI above the 15th percentile (Figure 2G,H).
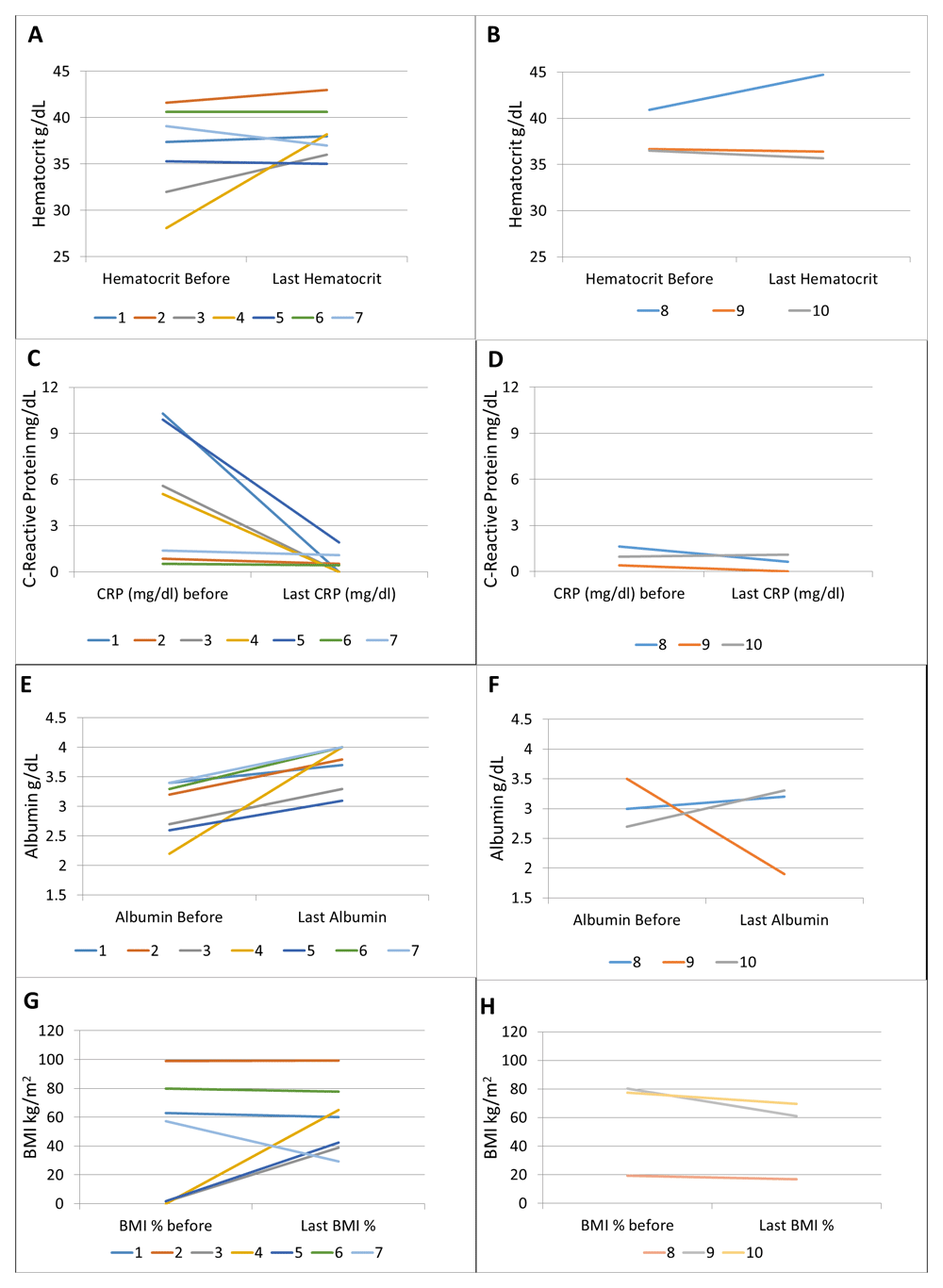
Hematocrit in patients with (A) augmented dosing and (B) Q8 dosing; CRP in patients with (C) augmented dosing and (D) Q8 dosing; albumin in patients with (E) augmented dosing and (F) Q8 dosing; BMI in patients with (G) augmented dosing and (H) Q8 dosing.
Among the seven patients, five had ustekinumab trough level showing low or undetectable drug level when receiving medication at a 6 or 8 week intervals (Table 2). Subsequently, four patients had escalation in frequency to Q4 and either achieved remission or clinical improvement. Following this change in interval, repeat drug levels for three patients were all therapeutic at 8, 7.1, and >10 μg/mL. Subsequently one of these three patients’ maintenance interval was decreased to Q6, and by the time of this study’s conclusion, a repeat level has not been obtained. Frequency was increased to Q6 in another patient, resulting in clinical remission. One of the patients was empirically started on a maintenance dose of Q4 interval and had trough levels of >10 μg/mL at 4 weeks. Frequency was subsequently changed to Q8, but a repeat drug level was not obtained (Table 2).
Three of the 10 patients were on standard ustekinumab dosing. One patient had symptomatic duodenal stricture and obstruction, resulting in abdominal pain, vomiting, and weight loss. He underwent endoscopic stricture dilation 2 months prior to initiation of ustekinumab. After ustekinumab was started, he required two subsequent dilations in a 2-month period, but had subsequently been in remission on high dose steroid. His hematocrit, CRP, and albumin all improved compared to levels prior to ustekinumab, while his BMI decreased slightly. The remaining two patients had disease worsening on ustekinumab, shown by serology and increasing abbrPCDAI. None of these three patients had their levels checked.
Complications observed while on ustekinumab included infusion reactions, such as low grade fever, joint pain and vomiting within one week of infusion, and infections such as Clostridium difficile, influenza, and pneumonia. Of note, one patient developed perianal abscess within a few weeks of the first ustekinumab induction, requiring hospitalization and resulting in stopping therapy. Upon the second induction more than 1.5 years later, he again developed forearm abscess requiring hospitalization. However, his CD went into remission with ustekinumab. Work-up for immune deficiency was negative. He was later diagnosed with maturity-onset diabetes of the young.
Here we report 10 pediatric patients with CD refractory to currently approved medications including anti-TNFs, immunomodulators and some to vedolizumab, with seven showing a clinical response to ustekinumab treatment. The majority of our patients showed positive response to ustekinumab within the first 2–3 months of therapy and remission by the time this study was concluded. Four of these seven patients had endoscopic data pre and post ustekinumab, out of which three showed an improvement as measured by SES-CD. In general, SES-CD score showed higher level of disease activity than abbrPCDAI, which is likely due to poor correlation between these two indices19. Moreover, abbrPCDAI and SES-CD information were collected at different times in the treatment course, resulting in small differences in disease activities. CRP, albumin, and BMI showed the largest improvement, and hematocrit improved in all but two patients who responded to treatment.
To achieve clinical response and/or remission, 7 out of 10 patients needed augmented maintenance doses. Of note, one among these seven patients, one (patient 7) initially achieved remission on standard Q8 dosing and SCD for 17 months. He had a disease flare when family liberalized his diet and failed to improve when he went back on SCD. He had no ustekinumab trough level during disease remission, but the most recent level of 1.1 μg/mL coincided with disease exacerbation and increase in maintenance frequency to Q6 resulted in clinical remission. More data from subsequent follow ups is needed to determine if disease activity corresponds to dosing frequency and trough level.
Among the three patients on standard dosing for the entire duration of treatment, only one achieved remission. He required two endoscopic dilations for duodenal stricture within 4 months of starting ustekinumab, but thereafter remained in remission for the next 5 months. However, this was confounded by his family continuing 60mg prednisone daily for at least 4 months (2 months longer than prescribed). Unfortunately, there was no subsequent follow ups as he had transitioned to adult care. He had ileocolonic as well as symptomatic gastroduodenal CD, which is a relatively rare manifestation and only affects about 2% of CD patients20. There are currently no well- established treatment protocols for gastroduodenal CD, and despite treatments with corticosteroid, 6-MP, ASA, and anti-TNF agents, 31% of patients eventually require surgery21. We cannot conclude if patient 7’s clinical improvement was secondary to ustekinumab or corticosteroids.
In the six patients on whom therapeutic drug monitoring (TDM) was performed, we found subtherapeutic drug levels on Q6 and Q8 dosing intervals, which corresponded with poorly controlled disease activities and all these patients showed clinical response to changing the dosing interval. On the two patients who were on standard dosing interval (Q8 weeks), TDM was not performed and thus we cannot determine if treatment failure was due to subtherapeutic dosing or a primary non-response to ustekinumab. A larger, randomized trial is needed to confirm the role of ustekinumab TDM in pediatric CD patients. Battat et al showed that over 75% of adult CD patients needed Q4 week dosing for maintenance of clinical response. This study also demonstrated a positive association of biomarkers and endoscopic improvement with ustekinumab trough levels >4.5 μg/mL18. In addition, in a case series of three adult CD patients, Park et al demonstrated an ability to recapture response by dose escalation among patients who lost response to standard ustekinumab dosing regimen22. Thus, based on our experience and existing literature, we recommend proactively checking trough levels 4 weeks after maintenance therapy initiation to guide dosing frequency early in the treatment course or to consider reactively checking levels and augmenting maintenance dosing interval in patients with sub-optimal or poor response to standard dosing.
Serious adverse effects were rare among our patients despite shorter dosing intervals. Only two patients developed recurrent infections and required hospitalization while on ustekinumab. Even though the patient who was hospitalized for abscesses also had other comorbidities such as acne, skin picking, psoriasis, and was previously hospitalized twice for recurrent abscesses on certolizumab, ustekinumab could not be ruled out as a cause of these infections. We did not observe any serious infections or cancers in the remaining eight patients, suggesting that ustekinumab is relatively well tolerated even at a higher frequency.
Our study is limited by its small size and retrospective nature. Furthermore, induction and maintenance doses were not uniform among all patients. TDM was only performed on six patients, and follow-up trough for five out of those six patients have not been obtained after changes in dosing frequency.
Only six patients had pre and post-treatment endoscopy, and two of those patients had intestinal surgeries, which might have altered SES-CD scores. Even though abbrPCDAI and other laboratory workups were helpful to correlate disease activities, fecal calprotectin should be added to further assess inflammation.
In this retrospective chart review, 7 out of 10 patients with anti-TNF refractory pediatric-onset Crohn’s disease required augmented maintenance doses of ustekinumab to achieve clinical response or remission as measured by abbrPCDAI. The remaining three patients on standard maintenance doses either did not respond or had confounding factors affecting clinical response. Further large randomized studies with closer therapeutic drug monitoring are needed to assess the relationship between dosing interval, trough levels, and clinical response in the pediatric population. Longer follow up is also needed to assess response once ustekinumab has reached therapeutic level.
Figshare: Table 1. Baseline characteristics of patients included in the study, https://doi.org/10.6084/m9.figshare.1204864523.
Figshare: Table 2. Ustekinumab dosing, interval, levels, duration of therapy, and complication, https://doi.org/10.6084/m9.figshare.1204863324.
Figshare: Table 3 Clinical response of anti-TNF refractory pediatric Crohn Disease patients on ustekinumab.csv, https://doi.org/10.6084/m9.figshare.1201260025.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The abstract of this work was presented at the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Annual Meeting (October 17-19, 2019, Chicago, IL; abstract 101).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric inflammatory bowel Disease
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Dayan J, Dolinger M, Benkov K, Dunkin D, et al.: Real World Experience With Ustekinumab in Children and Young Adults at a Tertiary Care Pediatric Inflammatory Bowel Disease Center. Journal of Pediatric Gastroenterology and Nutrition. 2019; 69 (1): 61-67 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Inflammatory bowel disease, Crohn's disease, ulcerative colitis, molecular genetics, epigenetics, nutrition, developmental origins of disease, recurrent Clostridioides difficile infection, fecal transplantation, microbiome, microbial therapeutics.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 Aug 21 |
read | |
|
Version 1 30 Apr 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)