Keywords
Coronavirus, COVID-19, Severe acute respiratory syndrome coronavirus 2, Autopsy, Diffuse alveolar damage, Acute respiratory distress syndrome
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
Coronavirus, COVID-19, Severe acute respiratory syndrome coronavirus 2, Autopsy, Diffuse alveolar damage, Acute respiratory distress syndrome
Coronavirus disease 2019 (COVID- 19) is a novel coronavirus that has spread rapidly across the globe and on the 12th March 2020, the World Health Organisation declared the outbreak a global pandemic1. The current data suggests the mortality rate from COVID-19 is 2–5%2. The spectrum of disease severity varies, with 14% suffering severe disease and 5% experiencing critical disease, defined as respiratory failure with shock or multi-organ dysfunction3. Respiratory failure is often the result of acute respiratory distress syndrome (ARDS)4. The current literature on the histopathological changes associated with COVID-19 infection is limited to ten cases and diffuse alveolar damage (DAD) is consistently reported as the primary abnormality5–9.
We present the case of a 65-year-old female who died in the community amid the COVID-19 pandemic. The aim of this report is contribute to the limited data available on the pathological findings in COVID-19 cases and to highlight the challenge of ascertaining virological PCR confirmation in those patients who have died.
A 65-year-old Caucasian female cleaner with a background of chronic kidney disease and morbid obesity contacted her GP regarding a short history of dry cough and a sore throat. Her GP prescribed antibiotics and advised the patient to self-isolate in line with the NHS COVID-19 guidance. The following day she was discovered deceased at her home address.
The Coroner requested a post mortem examination. External examination of the body confirmed morbid obesity (BMI 49.6kg/m2). There was no evidence of recent injury. On internal examination, the most striking abnormality was the appearance of the lungs, which were heavy (combined weight 1658g) and diffusely firm (Figure 1). No pus was expressed from the cut surface. The air passages contained frothy oedema and there was mild reddening of the mucosae. Additionally, there was cardiomegaly (heart weight: 524g) and both ventricles appeared dilated. Scattered, coarse scars were present across the renal surfaces. Internal examination showed no other significant abnormality.

(a) Both lungs were firm and heavy; the left lung weighed 778g and the right lung weighed 880g. (b) The cut surface of the lung appeared reddened. There was no consolidation or pus.
Histology of the lungs showed diffuse hyaline membrane formation, typical of DAD. In areas, narrow hyaline membranes lined the alveolar sacs; elsewhere, these changes were more severe (Figure 2 and Figure 3). There was prominent pulmonary oedema and variable congestion of the alveolar septal capillaries (Figure 4). Focally there was an increase in interstitial chronic inflammatory cells and there was type II pneumocyte hyperplasia (Figure 5 and Figure 6). In areas, the bronchiolar epithelium appeared attenuated with possible ulceration, indicating larger airway involvement (Figure 7). No viral inclusion bodies or multinucleated giant cells were seen.
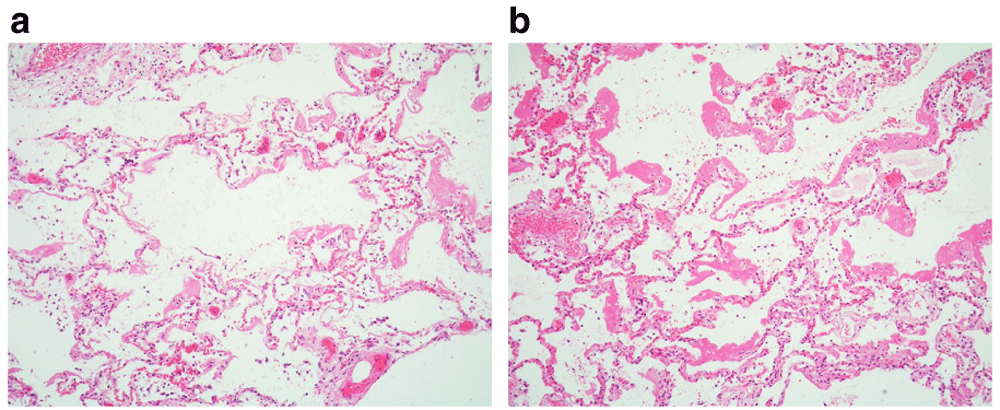
(a) The histologic features showed relatively marked variation; in some areas, the diffuse alveolar damage appears milder. Early hyaline membranes line the alveolar airspaces and ducts. (b). Elsewhere, hyaline membranes are broader and more extensive. (×20)
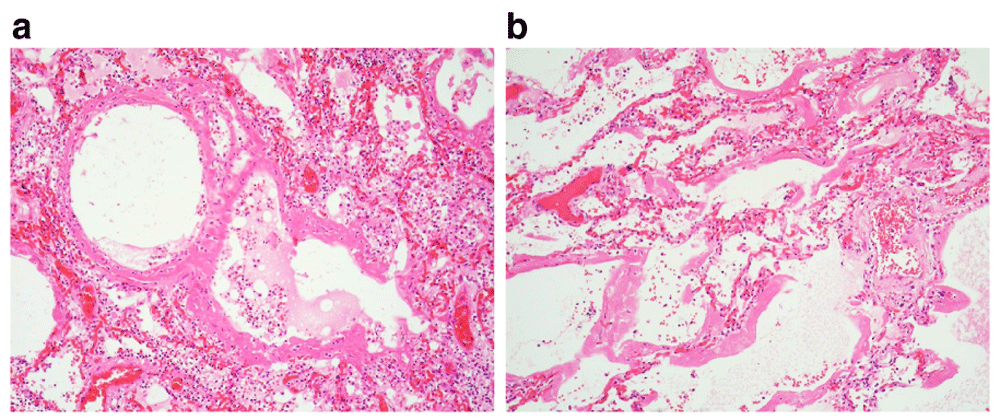
(a & b) The hyaline membranes are often regarded as the defining feature of the ‘exudative phase’ of diffuse alveolar damage. In the airspaces contained necrotic cellular debris and haemorrhagic oedema rich in fibrin due to the damaged alveolar walls. These materials condense to form the hyaline membranes. (x10, ×20).
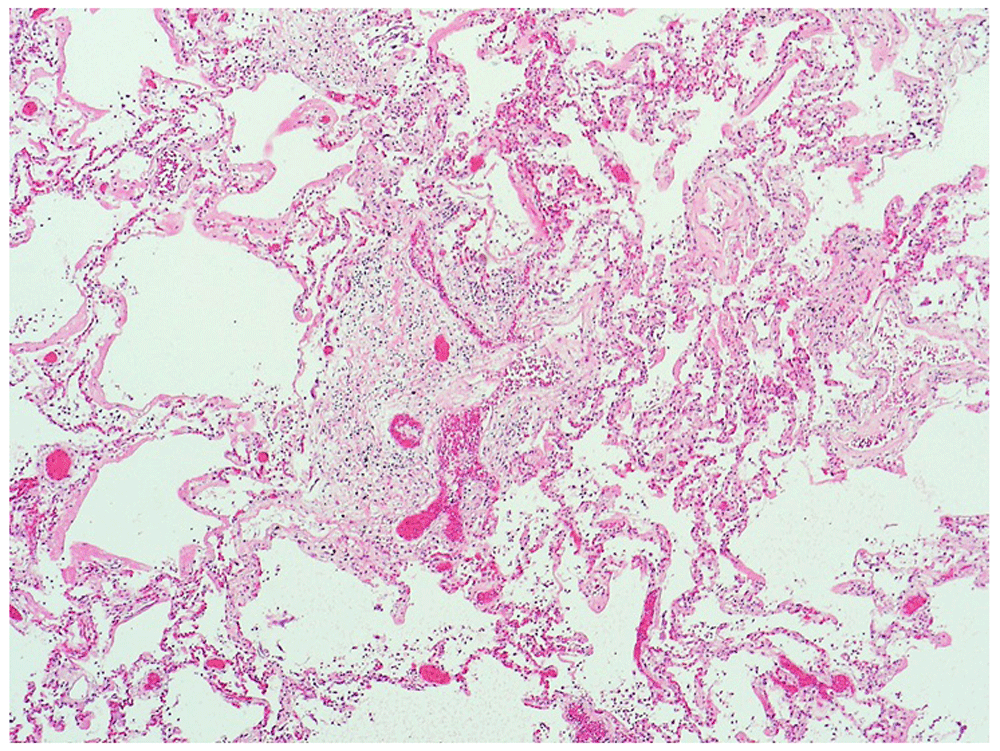
Very occasional neutrophils are present within the bronchiole walls, but no acute inflammatory cells are seen within the airspaces. Scattered, large cells with multilobed nuclei are present in the alveoli, which likely represent megakaryocytes [not visible at this magnification]. (x10).
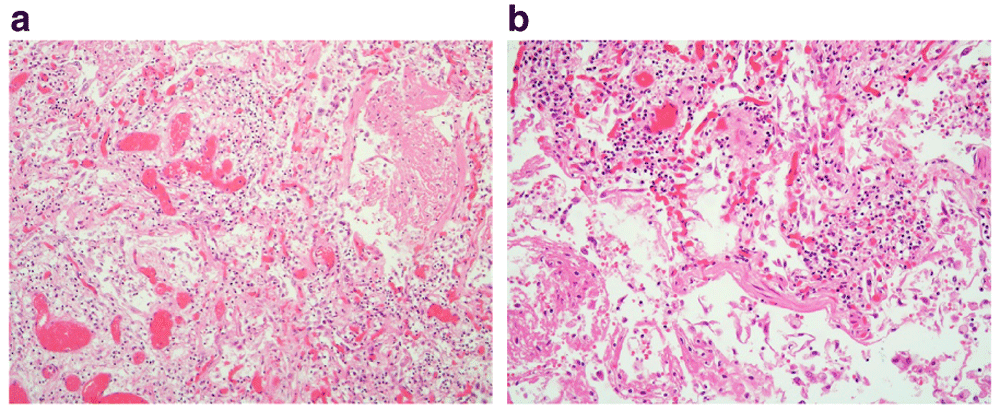
(a & b) There is hyperplasia of type II pneumocytes and expansion of the alveolar walls due to fibroblast proliferation. (x10, x20).
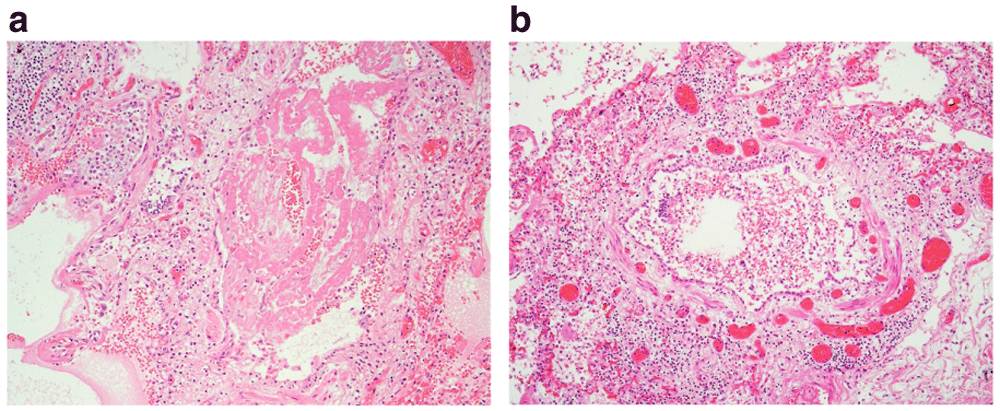
(a) There is widespread loss of the respiratory epithelium, which is likely artefactual and is often seen in post mortem lung histology; however, very occasionally this feature appears genuine. Here, the bronchiole lining appears attenuated and the lumen contained a fibrin plug. (b) There is attenuation of the epithelium with focal ulceration (12 to 3 o’clock position). The bronchiole wall and surrounding parenchyma are infiltrated by chronic inflammatory cells. (x10).
Histology of the heart, brain, spleen, thyroid and tonsils were normal. Sections taken from the kidneys revealed mild glomerulosclerosis. The liver showed mild fatty change. Unfortunately, in certain parts of the UK, laboratories are not processing virology samples in patients who have already died and therefore virological confirmation was not available. The cause of death was offered as COVID-19 based on the history and histopathological findings.
This case report describes the autopsy findings in an individual who died in the community whilst isolating with symptoms of COVID-19. The current literature on post mortem findings in cases of COVID-19 infection is limited, but the available data consistently highlights DAD as the predominant pathological process. This case report contributes to the growing body of evidence that describes features of DAD in the setting of COVID-19 associated acute respiratory symptoms. There is also emerging evidence to suggest some individuals may develop cardiovascular complications due to COVID-19 infection10–12. In this case, the heart was enlarged but histology was normal and these changes most likely represent obesity-related cardiomegaly.
The absence of post mortem virology testing is a limitation in this case. Considering the consistent symptomology and amidst the current pandemic, the histopathological changes were considered diagnostic for COVID-19. Availability and sensitivity of testing seem varied across the UK. In some regions, autopsy examinations are delayed whilst awaiting results from post mortem nasal swabs; an invasive examination is only carried out if these results are negative. Interestingly, in the recent case published by Barton LM et al.,9 nasal swabs were positive for COVID-19 but pulmonary swabs were negative and post mortem examination revealed bronchopneumonia as the cause of death, suggesting post mortem nasopharyngeal swabs may not be an accurate screening method after death. Based on the data available, it appears that lung histology provides the most reliable means to attribute death to COVID-19 infection, providing the clinical history is appropriate. It is worth noting that currently in the UK, suspected COVID cases in the community are not able to access confirmatory swabbing and so the onus will often fall to the pathologists to establish an appropriate cause of death. From anecdotal hospital experience, patients who develop COVID-19 associated ARDS will often succumb rapidly following a dramatic increase in oxygen requirements over a period of several hours (Johnson O, personal communication, 14 Apr 2020). For this reason, the authors are aware that the number of community deaths will inevitably continue to increase given that some patients may not be able to get to secondary care in a timely fashion.
At our centre, invasive autopsies in cases of suspected COVID-19 infection are now only performed when the history indicates a probable alternative to the cause of death, or where there are concerns that the death may be suspicious. When it seems that death has resulted from respiratory failure, post mortems are limited to an external examination for the purposes of ruling out unnatural causes. Providing there are no unexpected or suspicious findings, a cause of death is offered based on a history consistent with COVID-19 infection. Whilst there are many benefits to an invasive autopsy, such as facilitating accurate diagnosis, excluding internal trauma and improving understanding of the disease, factors such as accessibility of COVID-19 testing, availability of personal protective equipment, infection risk to staff and body storage capacity must be considered. Where COVID-19 is suspected, the appropriateness of performing an invasive procedure must be decided on a case-by-case basis.
Written informed consent for publication of their clinical details and clinical images was obtained from the next of kin of the patient.
All data underlying the results are available as part of the article and no additional source data are required.
The authors would like to thank HM Coroner for the Liverpool and Wirral area for his permission to publish this case. The use of this case is in accordance with the requirements of the Human Tissue Act 2004 (UK).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pulmonary pathology
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Morbid anatomy and infectious diseases.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 May 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)