Keywords
Arabidopsis, floral dip, transformation
Arabidopsis, floral dip, transformation
The updated version of this manuscript includes additional references on floral dip transformation in the introduction section, clarification of data in Figure 1 in the legend, and addition of the OD600 value of the Agrobacterium cell density at the time of harvest in the Method section.
See the authors' detailed response to the review by Zhanyuan J Zhang
See the authors' detailed response to the review by Ying Wang and Shelley Hepworth
Plant transformation integrates foreign genes into the plant nuclear genome. The development of different transformation protocols in various plants has enabled advances in plant molecular biology and crop improvements (Saifi et al., 2020). Agrobacterium is routinely used as a plant gene transformation vehicle as it naturally possesses the ability to transfer a segment of its plasmid DNA (T-DNA) into its host nucleus, which ultimately leads to integration of the T-DNA into the nuclear genome (Tzfira et al., 2004). During 1980s and early 1990s, generating transgenic plants by leaf disc-based Agrobacterium-mediated transformation required laborious plant tissue culture and regeneration steps. In 1993, a simple floral vacuum infiltration method was developed in Arabidopsis for stable transformation, overcoming the tedious tissue culture requirements (Bechtold et al., 1993). Later, the vacuum infiltration step was replaced by floral dipping where the developing floral tissues are dipped into a solution containing Agrobacterium, sucrose and the surfactant Silwet L-77 (Clough & Bent, 1998). Because of the simplicity and reliability of this floral dip method, it is now the commonly used transformation method in Arabidopsis. This protocol has also been shown to work in certain Brassicaceae plants (Bent, 2006). Floral dip transformation may be feasible in plants such as wheat, maize, tomato, flax, Medicago truncatula and Setaria viridis (Agarwal et al., 2009; Bastaki & Cullis, 2014; Martins et al., 2015; Mu et al., 2012; Trieu et al., 2000; Yasmeen et al., 2009).
In Agrobacterium-mediated transformation protocols, the concentration of bacterial inoculum has been considered crucial to the success of plant transformation. In the commonly used floral dip protocol, bacterial cells are grown to stationary phase (OD600=2.0), pelleted and resuspended in inoculation medium to OD600≥0.8 (Clough & Bent, 1998; Zhang et al., 2006). Here, we tested whether a low concentration of Agrobacterium inoculum affects the plant transformation rate. Our data showed that, contrary to our expectation, using an extremely low density of Agrobacterium inoculum (OD600=0.002) in the floral dip method still warrants relatively high transformation rate in Arabidopsis.
Arabidopsis Col-0 wild type plants were grown in a growth room under long day (16 h light/8 h dark cycle) at 23°C. Seedlings were grown at a density of 30–40 per 64 cm2 (8 cm × 8 cm) pot in moistened potting soil initially and transplanted to 64 cm2 pots with eight plants per pot when they were two weeks old. After plants bolted and floral buds are formed (~30-day-old), they were used in floral dip transformation.
The plasmid pCambia1305-3flag-NOS was transformed into Agrobacterium tumefaciens strain GV3101 (Van Larebeke et al., 1974) by mixing the plasmid DNA with the bacterial cells in a 1mm gap cuvette (BTX, #45-0124) followed by electroporation for 5 millisecond at 1,500 volts using the ECM 399 Electroporation System (BTX, #45-0000) (Gao et al., 2009). The resulting strain was used in the plant transformation experiments. Bacteria were grown overnight in sterilized 4 ml LB media (Bio Basic Inc., #SD7002) with kanamycin, gentamicin and rifampicin antibiotics (50 μg/ml each, Bio Basic Inc. #KB0286, #GB0217, #RB0808) in a 28°C shaker (New Brunswick Scientific Co G25 Controlled Environment Incubator Shaker). Then the overnight culture was diluted into 100 ml LB media with kanamycin (50 μg/ml) and allowed to grow further for 8 h (OD600=1.5~1.8) in the same shaker. The bacteria were collected by centrifugation (Thermo Scientific, Sorvall Legend X1R) at 6000 g for 10 min at room temperature and then resuspended in 100 ml floral dip medium to final OD600 of 1, 0.1, 0.01, and 0.002 (measured by BioSpec-1601 UV-visible spectrophotometer from SHIMADZU) prior to use. The floral dip medium contained 5.0% (w/v) sucrose (Bio Basic Inc. #SB0498) and 0.01% (v/v) Silwet L-77 (PhytoTechnology Laboratories #S7777) in distilled water.
For floral dip, pots were tilted and floral buds were submerged in bacterial suspension with 30 sec of gentle agitation. The dipped plants were then covered with a tall clear-plastic dome to maintain humidity. Plants were placed in a dark room overnight before being moved back to the growth room. The domes were removed approximately 48 h after the floral dip treatment. Plants were grown for another 30–32 days until siliques became brown and dry. Each pot with 8 plants were transformed separately. For each concentration of Agrobacterium inocula, 4–6 pots of plants were transformed depending on the number of plants available for transformation in each experiment, this varied mainly due to the uneven germination of the seeds in each experiment. About 6000 seeds were bulk harvested from the plants grown in a pot. Seeds were harvested by gentle stripping of dried inflorescences by fingers above a piece of clean paper. The debris from the stem and pods was removed from the seeds by gentle blowing. Seeds were kept in a 37°C incubator for two days for desiccation.
Prior to selection, seeds were surface sterilized with 20% (v/v) bleach (Clorox Regular Bleach) containing 0.1% (v/v) Tween20 (Sigma-Aldrich #P1379) for 1min, followed by three times rinse with sterile water. The sterilized seeds were suspended in 0.1% (w/v) sterile agar (Bio Basic Inc. #FB0010) and plated on hygromycin selection plates (1/2 MS medium, Murashige & Skoog Basal Medium with Vitamins from PhytoTechnology Laboratories #M519 and 50 μg/ml hygromycin, Bio Basic Inc. #HD0230) at a density of approximately 3000 seeds (0.06 gram by weight) per 92×12mm (diameter×height) petri plate (Sarstedt #82.1473.001). Seeds collected from each pot (4–6 pots for each concentration of Agrobacterium inocula) were plated on a separate selection plate. Plates were placed in 4°C refrigerator for two days before moved to a plant growth chamber (16 h light/8 h dark cycle, Conviron Model A1000). The plants were grown at 23°C for 10 days before transformants were identified as hygromycin-resistant seedlings that produced green leaves and well-established roots grown on the selective medium. The experiment was repeated three times by transforming independently grown plants with different concentrations of Agrobacterium inocula.
Four Agrobacterium inocula from high to low concentrations (OD600=1, 0.1, 0.01, 0.002) were used in floral dip transformation to test the effect of bacterial concentration on the transformation rate. As shown in Figure 1 (Underlying data (Wang, 2020)), similar transformation rate (approximately 0.60%) was observed under all tested bacterial concentration. Notably, the transformation efficiency remains unchanged even though the Agrobacterium inoculum was diluted 500 times form OD600 = 1 to OD600 = 0.002. Therefore, it is feasible to dramatically reduce the Agrobacterium inoculum concentration in the floral dip method. Regardless of the inoculum concentration, transforming eight Arabidopsis plants grown in a single pot produced about 36 T1 transgenic lines on average, which is sufficient for most studies.
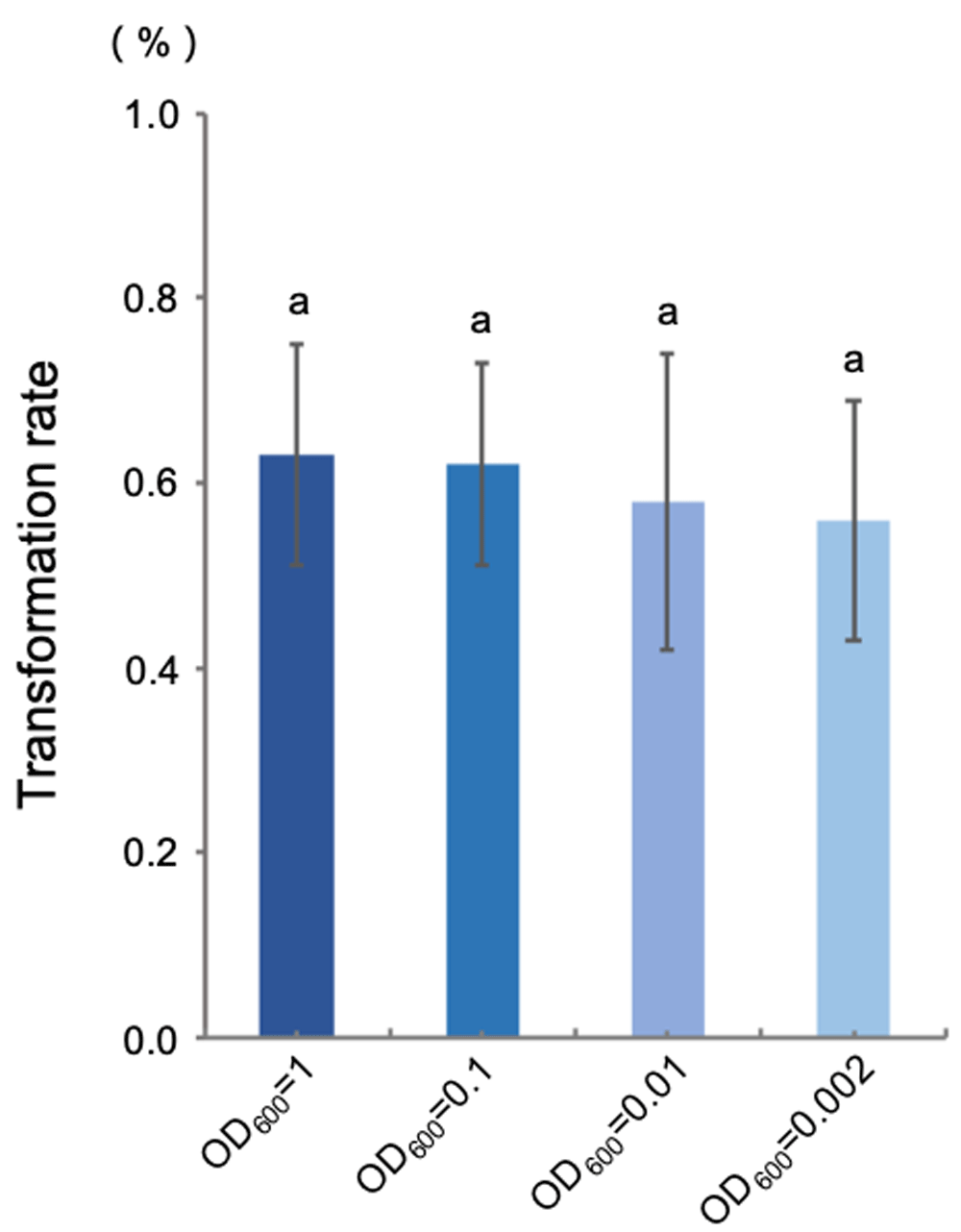
Transformation rates were calculated as [(# of hygromycin-resistant seedlings) / (total # seedlings tested)] × 100%. The data are shown as mean ± SE from six independent repeats in one representative experiment. The same letters denote no statistically significant difference according to one-way ANOVA (p<0.05).
Standard floral dip protocols use high concentrations of Agrobacterium inoculum, which requires growing large bacterial cultures (Clough & Bent, 1998; Zhang et al., 2006). Our study showed that Agrobacterium inoculum can be diluted to as low as OD600 = 0.002 without sacrificing the transformation efficiency. Thus, the volume of bacterial culture used in each transformation experiment could be greatly reduced. For example, diluting 0.1 ml of overnight culture (OD600 = 2) to OD600 = 0.002 gives ~100 ml bacterial inoculum, which is sufficient in most transformation experiments. Such improvement allows researchers to culture small volume of a large number of Agrobacterium strains in parallel and use the diluted cultures to carry out high-throughput transformation of a large number of different constructs into Arabidopsis plants.
Open Science Framework: High transformation efficiency in Arabidopsis using extremely low Agrobacterium inoculum project.
https://doi.org/10.17605/OSF.IO/YF6AE (Wang, 2020)
This project contains the following underlying data:
Transformation efficiency.xlsx (raw data of results from transformation using different Agrobacterium concentrations)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant tissue culture and transformation and plant molecular biology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant development, Arabidopsis thaliana, molecular genetics, flowering, meristems, transcription factors, gene expression, transgenic plants
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 Sep 20 |
||
|
Version 1 13 May 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)