Keywords
peptic ulcer disease, upper gastrointestinal bleeding, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, children
peptic ulcer disease, upper gastrointestinal bleeding, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, children
In the new version, revisions in various sections have been made following the reviewers recommendations.
Introduction – As suggested by the reviewers, regarding clarity of information related to epidemiology, we elaborated more about the indications for endoscopies mentioned in the two studies to which we referred in the second paragraph of the first version.
Case presentation – Following the reviewers recommendation, we added hemodynamic parameters at admission (first paragraph), we gave more details of drugs administration (first and second paragraphs), family history and relevant medical history (second paragraph) and nutritional status of the patient (third paragraph).
Discussion- We expanded on the accumulation of risk factors (third and fourth paragraphs), including family history, NSAIDs and paracetamol administration in the presented case, referring to the data found in published literature.
See the authors' detailed response to the review by Vera L. Sdepanian
See the authors' detailed response to the review by Parijat Ram Tripathi
Peptic ulcer disease (PUD) affects 1–2/1000 people annually in the USA, UK and Europe and has been gradually decreasing1. An explanation could be the declining prevalence of Helicobacter pylori infection. While the rate of infections is decreasing, the rate of complications remains static, likely due to an aging population which has an elevated usage of ulcerogenic medication1.
PUD occurs less frequently in children than adults. Epidemiological data are limited due to the rareness of the disease. A prospective European multicenter study aimed at determining frequency and risk factors of gastric and duodenal ulcers in children who underwent upper gastrointestinal endoscopies for different indications (epigastric or abdominal pain, gastroesophageal reflux disease)2. The study showed a frequency of 8.1% of ulcers and/or erosions, occurring mainly during the second decade of development2. In the USA, another study reported 17.4% of insured pediatric patients diagnosed as having any upper gastrointestinal ulcer developed peptic ulcer bleeding3.
PUD is a heterogenous disease defined by an imbalance between mucosa-protective and aggressive factors in the presence of risk factors including: H. pylori infection, chronic disease (inflammatory bowel disease, rheumatic diseases) and drug use, particularly nonsteroidal anti-inflammatory drugs (NSAIDs)2. In practice, NSAIDs are commonly used to manage acute febrile illness or pain in healthy children. One adverse reaction is acute gastrointestinal bleeding associated with short-term NSAIDs use, with a high rate of hospitalization and mortality in developed countries4. The adverse effect of short-term utilization of NSAIDs among children and their association with PUD are less clear.
We present a rare case of upper gastrointestinal bleeding following a low dose of ibuprofen in a 3-year-old to underline potentially severe side-effects of short-term NSAIDs use at appropriate doses in children.
A 3-year-old-female, with a family history of peptic ulcers, was admitted with fever, coffee-ground vomiting and abdominal pain, hemodynamically stable (heart rate 128 beats per minute, blood pressure 108/71 mmHg, respiratory rate 28 breaths per minute). The mother stated the patient received two weight-appropriate doses of ibuprofen (two doses of 100 mg -6.66 mg/kg- by mouth, 8 hours apart) and a dose of paracetamol (250 mg - 16.66 mg/kg- by mouth), both administered within an appropriate time interval in the previous 24 hours for fever control.
The patient had a positive medical history of upper respiratory tract infections with febrile seizures and interstitial pneumonia treated with antypiretics and clarithromycin, respectively. In the first days of the upper respiratory infection (5 weeks prior to the bleeding episode) ibuprofen 100 mg -6.66 mg/kg- was administered by mouth every 8 hours for three days and in the next two days two doses a day within an appropriate time interval. For the convulsion episode no antiseizure medication was needed. Also, clarithromycin 7.5 mg/kg/day was administered by mouth for 10 days. The duration of the symptoms was 2 weeks.
The patient is allergic to cephalosporin and amoxicillin/clavulanic acid. No immune deficiency disease was documented.
Clinical examination revealed general malaise, pallor, fever, pharyngotonsillar congestion and productive cough, normal breath sound, a distended and mildly tender abdomen moving normally with respiration and normal stool. The patient weighed 15 Kg (z-score 0.65) at the 72nd percentile and measured 88 cm tall (z-score -1.63) at the 5th percentile for stature. She has a body mass index (BMI) of 19.4 (z-score 2.15), placing the BMI-for-age at the 98th percentile.
Initial laboratory tests indicated anemia with reticulocytosis (Hematocrit 29.7%, Hemoglobin 9.6 g/dl, reticulocytes 3.6%, corrected reticulocyte count 3.24) and lower total protein (5.52 g/dL). Remaining laboratory results were normal, including coagulation tests.
Soon after hospitalization, the patient had a second episode of coffee-ground vomiting.
An upper digestive endoscopy with biopsy was performed revealing a non-bleeding gastric ulcer at 2 cm from pylorus (Figure 1). H. pylori gastric biopsy testing was negative.
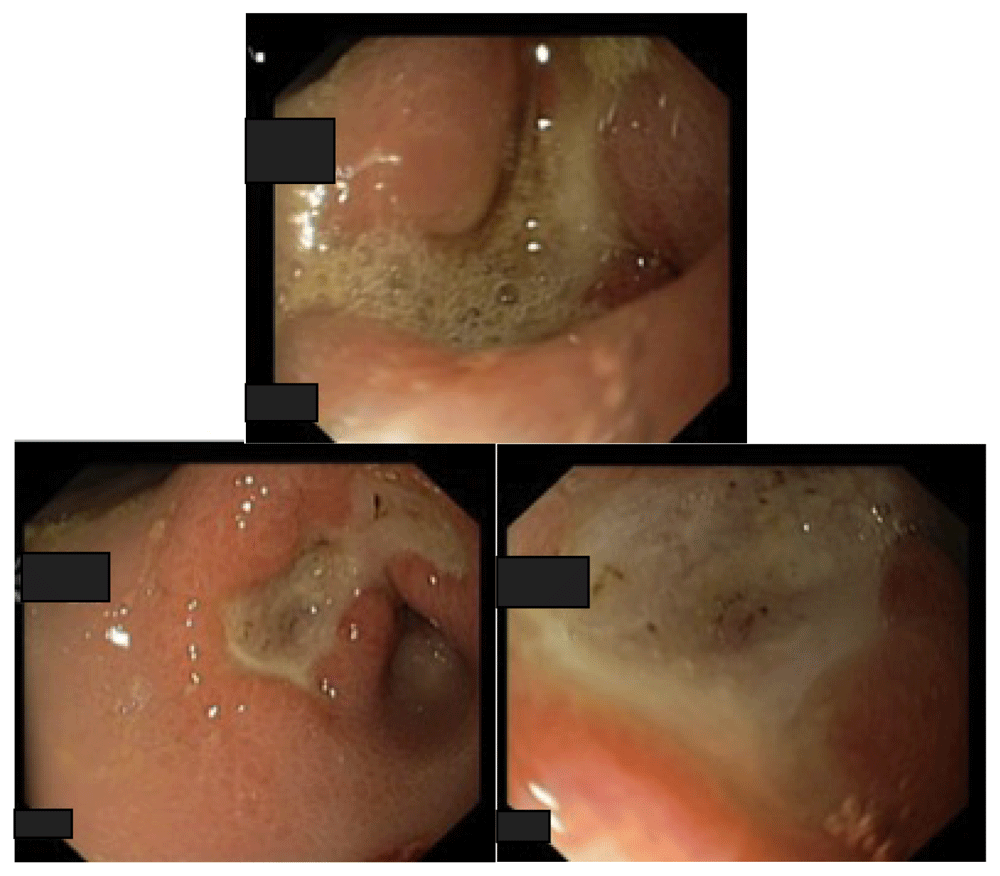
This shows a non-bleeding gastric ulceration measuring 2.5 × 2 cm with edematous rim located 2 cm from the pyloric ring; pale gastric mucosa, fluid stasis and food debris; snake skin appearance of gastric mucosa in the fundus.
Based on this data, a diagnosis was made of NSAID-induced gastric ulcer, causing upper gastrointestinal bleeding.
During hospitalization, perfusion with glucose and electrolytes was administered in order to compensate for fluid loss. The patient was treated with a proton pump inhibitor (esomeprazole 10 mg/day - 0.66 mg/kg/day) for 2 months.
There were no further gastrointestinal symptoms. Hemoglobin values returned to normal, indicating resolution of gastrointestinal bleeding and the report of the endoscopy performed at the end of the treatment period confirmed the healing of the gastric ulceration area.
Upper gastrointestinal bleeding in a 3-year-old following short-term NSAIDs use is an uncommon presentation. Similar cases5 have been reported in literature, but the adverse effects of short-term NSAIDs use among children and their association with PUD is not completely understood. However, some studies offer compelling data indicating certain risk factors, primarily: the child’s age2, NSAIDs consumption2,4,6,7 and H. pylori infection2,6–8.
PUD seems to primarily affect patients between 10–20 years old2. A retrospective cohort study reported a lower median age for those with gastric ulcers, than those with duodenal ulcers8.
The second important factor is NSAIDs consumption. The probability of PUD increases with the duration of therapy, dose and presence of risk factors, including positive familial history or drugs coadministration7,9. Thus, despite a low dose of ibuprofen, the gastric ulcer (GU) in this case can be explained in part by a positive family history and association with a dose of paracetamol. A joint effect of paracetamol (a dosage higher than 2g) combined with NSAIDs was reported in adults that had both compounds prescribed together10. Conversely, the risk for gastrointestinal ulcers and ulcer complications due to normal paracetamol intake has not been yet supported by available biological and clinical data.
The accumulation of more risk factors, like positive family history, NSAID administration or H. Pylori infection in pediatric population affected by PUD, has been well documented. The father of the patient was diagnosed with PUD, but was unable to confirm whether he was H. Pylori positive.
Moreover, some studies conclude that short-term NSAIDs use is highly correlated with GU6. The association between short-term NSAIDs use and proton pump inhibitors (PPIs) can theoretically reduce the risk of upper gastrointestinal bleeding in children. Although coadministration of NSAIDs and PPIs is considered safe to reduce adverse gastrointestinal effects in adults11, there is not sufficient data about this drugs association in the prevention of short-term NSAIDs-PUDs in children.
The third important risk factor in PUD, H. pylori infection, was negative in our case. Some studies suggest a weaker association between H. pylori and PUD in children as compared with adults2,12. However, this infection is a well-recognized cause of chronic gastritis and plays an important role in the pathogenesis of PUD in children13.
Patients who develop gastrointestinal bleeding caused by NSAIDs-associated ulcers should discontinue use. Therapeutic strategies in these cases depend on the severity of presentation. Pharmacologic, endoscopic and surgical techniques have been developed to achieve hemostasis. In cases of massive bleeding, immediate endoscopic or surgical intervention is required. Scoring systems for upper gastrointestinal bleeding in children, laboratory tests and blood transfusion requirements are still under development14–16. In the present case, clinical presentation with two episodes of isolated hematemesis (coffee-ground vomiting) and endoscopic examination findings (non-bleeding gastric ulcer) correlated with laboratory tests indicated pharmacologic management.
Short term NSAIDs use in appropriate doses, commonly prescribed to control fever in children, can lead to PUD. Before administration, risk factors such as other antipyretic medication use, or a suggestive familial history must be considered. Doctors should inform caregivers of the risks involved and encouraging limited NSAIDs use.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for the publication of this case report was obtained from the parents of the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Gastroenterologist
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric gastroenterology and hepatology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 Jun 21 |
||
|
Version 1 22 May 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)