Keywords
Foveola gastrica, villous intestine, coconut shell, mangrove wood, rice husk, and kernel palm shell
Foveola gastrica, villous intestine, coconut shell, mangrove wood, rice husk, and kernel palm shell
In response to comments from the reviewers, the following is our amendments:
Abstract
See the authors' detailed response to the review by Srikanta Samanta
Trevally fish are a commercially important group of marine fish in the family Carangidae. A total of 146 species of trevally have been recorded worldwide1. These fish are distributed in tropical, subtropical, and temperate waters2–7. In Indonesia, trevally fish are found in the Aceh waters8,9, East Borneo10, Papua and Wester Nusa Tenggara11,12, and Java13.
Giant trevally, Caranx ignobilis, is among the most popular trevally fish in Indonesia. The population of this species has declined over the years due to overfishing7,14–16. Culture of this fish has been initiated in Aceh Province, Indonesia. However, farmers are faced with a feeding obstacle. Giant trevally in culture systems are currently fed waste fish and a commercial diet (Hi-Pro-Vite, Central Proteina Prima Company). The commercial diet is costly and difficult to obtain in remote areas, and the waste fish supply is very seasonal. Trash fish are limited in nutrients, particularly the essential amino acid composition17. Therefore, it is crucial to formulate a diet for giant trevally using local raw materials with higher protein, that is inexpensive, easy to find, and digestible.
Activated charcoal is commonly added to the diet to increase digestibility and trigger growth in fish. For example, Jahan et al.18 successfully used activated charcoal to increase the digestibility and growth performance of river catfish, Pangasiaodon sp. Other researchers have used charcoal in the diets of fish species, such as Nile tilapia, Oreochromis niloticus19–21, tiger pufferfish, Takifugu rubripes22, Japanese flounder, Paralichthys olivaceus23, African catfish, Clarias gariepinus24,25, gilthead seabream, Sparus aurata26, and sturgeon, Huso huso27. Firdus et al.28 added rice husk charcoal to the diet of giant trevally. However, the effect of charcoal on the morphology of the gut and intestine has not been reported.
Organogenesis of the digestive system occurs as fish age, and this process is strongly dependent on the quantity and quality of food29–32, which is related to the development of mucosal cells, amplification of apical plasma membranes, and formation of the foveola gastrica and intestinal villi33,34. It has been hypothesized that adding activated charcoal to the diet triggers the digestive organogenesis system process35,36. In this study, we tested four charcoal sources in the diet to evaluate the morphology of the gut and intestine of giant trevally. Information on the gut and intestinal morphology is important to understand the absorption mechanism of nutrients from the diet.
The study was conducted at the Center for Brackish Water Aquaculture, Ujung Batee, Aceh, Indonesia from February to July 2018. The activated charcoal was characterized at the Integrated Laboratory of Calibration, Universitas Gajah Mada, Yogyakarta, Indonesia. Histological samples were prepared at the Laboratory of Histology, Faculty of Mathematics and Natural Sciences, Universitas Syiah Kuala, Banda Aceh, Indonesia.
A completely randomized experimental design with five treatments consisting of control and four different charcoal sources was used in this study. The experimental groups were: (A) the experimental diet without charcoal, (B) the experimental diet with 2% charcoal from coconut shell, (C) the experimental diet with 2% charcoal from mangrove wood, (D) the experimental diet with 2% charcoal from rice husk, and (E) the experimental diet with 2% charcoal from kernel palm shell. All treatments were performed with four replications.
A total of 300 giant trevally juveniles of mixed sex (average body weight, 16.52 ± 3.12 g; total length, 10.28 ± 0.64 cm) were purchased from a local farmer in Lancang Barat Village, Aceh Utara District, Aceh, Indonesia. The fish were acclimatized in ponds (ponds size 2 m x 1.8 m and temperature of around 29°C) at the Center for Brackish Water Aquaculture, Ujung Batee for 2 weeks. The fish were fed an experimental diet containing 50% crude protein twice daily at 7 AM and 5 PM at 3% of body weight per day (Table 1).
The raw coconut shells, mangrove wood, rice husks, and kernel palm shells were chopped and ground. Approximately 500 g of the ground materials were placed on aluminum foil and heated in a furnace at 400°C for 1 hour. Nitrogen gas was flowed into the furnace to remove the oxygen. Then, the temperature was decreased to 30°C gradually and held for 1 hour. After 1 hour, the charcoal was removed from the furnace, sieved through a No. 40 mesh, and held in a jar before activating. A total of 100 g of sieved charcoal was taken and mixed with 400 ml of 0.2 M citric acid. The solution was stirred for 24 hours. After 24 hours, the solution was filtered through filter paper. The filtered charcoal was washed with distilled water and dried in an oven at 110°C for 24 hours.
The experimental diet was formulated from both plant and animal-based protein sources, such as Ebi-shrimp meal, fish meal, blood meal, soybean meal, rice flour, and corn flour. All raw materials were subjected to a proximate analysis before use in the formulation. Three types of amino acids i.e. isoleucine, L-tryptophan, and DL-methionine were also added (Table 1). A total of 2% of the tested charcoal sources was added to the formulation (Table 1). The formulated diets were subjected to a proximate analysis before use in the experiment.
The fish was captured randomly, measured for body weight and total length, and then distributed into 20 plastic containers (48 × 43 × 70 cm) at a stocking density of 15 fish per container. The water volume in the container was 75 L. The fish were fed an experimental diet twice daily at 7 AM and 5 PM to satiation for 42 days.
Gastric and intestinal samples were collected at the end of the study. Three fish from each treatment were taken randomly from the experimental tanks. The fish were anesthetized with 30 mg L−1 clove oil37, and the abdomen of the fish was gently dissected following the procedure of Purushothaman et al.38. The stomach and intestines were removed with scalpel scissors and preserved in 4% formalin for 1 week. Histological sampling was carried using the paraffin method based on Osman and Caceci39. The samples were dehydrated through an alcohol series and cleared in xylol. Subsequently, the gut and intestine samples were embedded in paraffin. The paraffin block was sectioned to 6 µm, and the sections were stained with hematoxylin and eosin. The size (height and width) of villi was determined using a binocular microscope (Zeiss Primo Star, Carl Zeiss Suzhou Co., Ltd., Suzhou, China) which was connected to a CCD camera and computer monitor19. All efforts were made to lessen harm to the animals by complying to the guidelines of ethics animal use in research of Syiah Kuala University.
The qualitative gut and intestinal morphology data were subjected to one-way analysis of variance followed by Duncan’s multiple range test. The analysis was performed using SPSS ver. 18.0 software. The qualitative (histological) gut and intestinal data were analyzed descriptively. A P-value < 0.05 was considered significant.
Adding activated charcoal to the diet significantly affected the length and width of the foveola gastrica and intestinal villi (P < 0.05). In general, fish fed the activated charcoal diets produced better results than those not fed the charcoal (Figure 1 and Figure 2). The best foveola gastrica morphology was obtained with the rice husk charcoal and the mean length and width of the foveola gastrica were 311.811 µm and 241.786 µm, respectively; followed by coconut shell charcoal (257.040 µm and 183.816 µm), kernel palm charcoal (229.969 µm and 169.131 µm µm), and mangrove wood charcoal (229.595 µm and 166.509 µm).
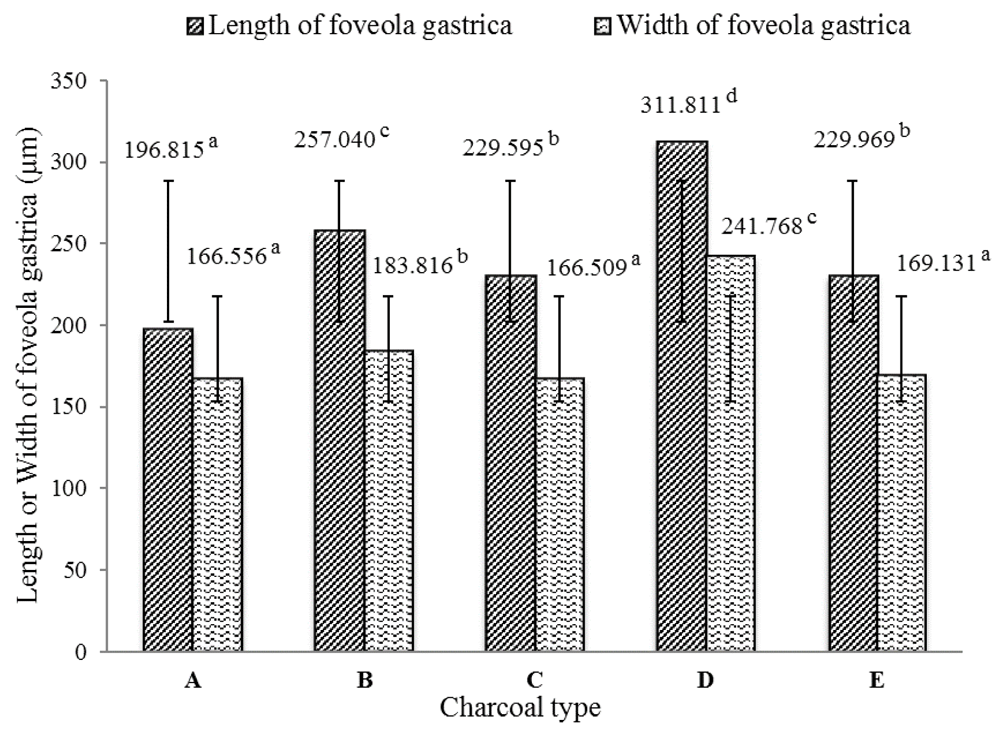
(A) Diet without charcoal, (B) diet with coconut shell charcoal, (C) diet with mangrove wood charcoal, (D) diet with rice husk charcoal, (E) diet with kernel palm shell charcoal.
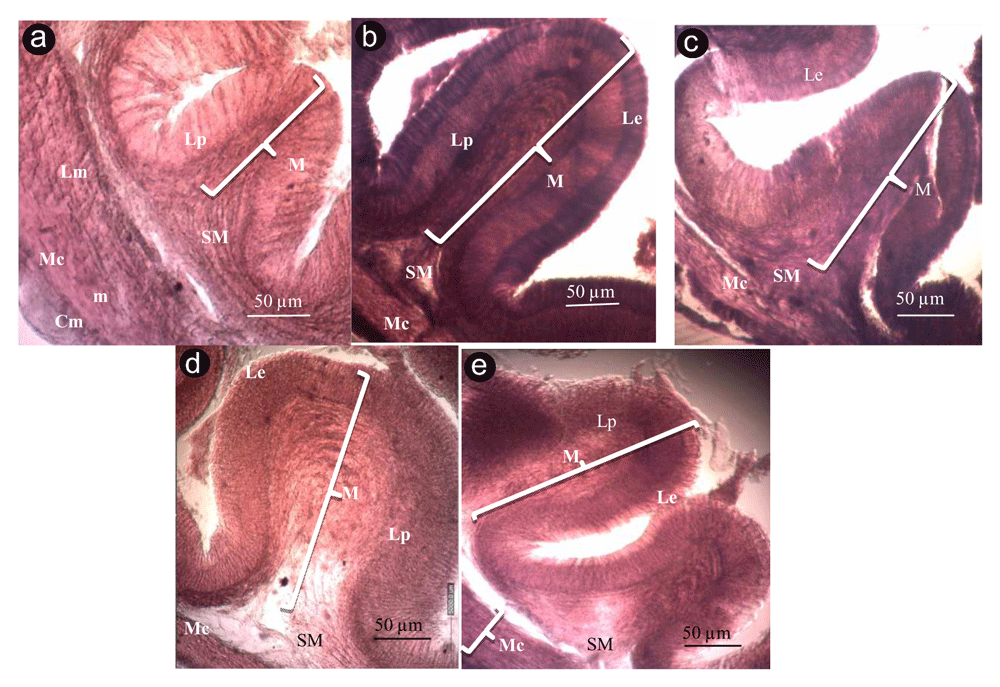
(A) Diet without charcoal, (B) diet with coconut shell charcoal, (C) diet with mangrove wood charcoal, (D) diet with rice husk charcoal, (E) diet with kernel palm shell charcoal. M, tunica mucosa; SM, tunica submucosa; Mc, tunica muscularis; Le, lamina epithelialis; Lp, lamina propria; m, muscle; Lm, longitudinal muscle fibers; Cm, circular muscle fibers (Cm).
The greatest length of the villous intestine was recorded in fish fed a diet with activated charcoal than those not fed the activated charcoal (Figure 3). The greatest growth of intestinal villi was determined in the mangrove active charcoal (mean, 135.012 µm) group, but this value was not significantly different from the rice husk or kernel palm shell charcoals (Figure 4). However, the greatest intestinal villi width was obtained in the treatment without activated charcoal (38.341 µm), and this value was significantly different from the other treatments.
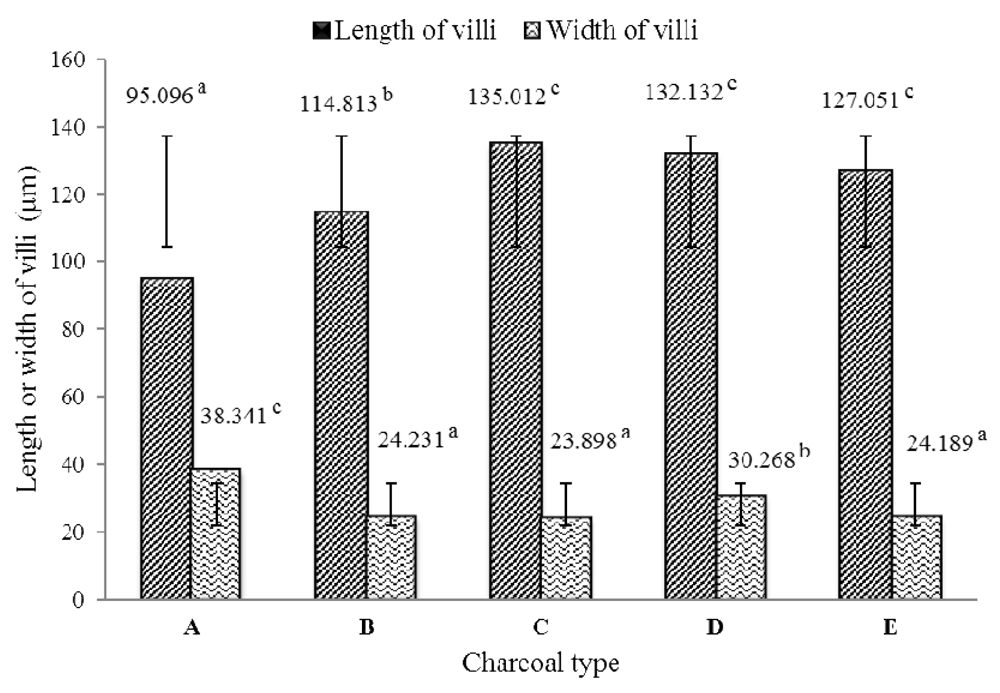
(A) Diet without charcoal, (B) diet with coconut shell charcoal, (C) diet with mangrove wood charcoal, (D) diet with rice husk charcoal, (E) diet with kernel palm shell charcoal.

(A) Diet without charcoal, (B) diet with coconut shell charcoal, (C) diet with mangrove wood charcoal, (D) diet with rice husk charcoal, (E) diet with kernel palm shell charcoal. M, tunica mucosa; SM, tunica submucosa; Mc, tunica muscularis.
Raw biometic data, in addition to unprocessed imaged, are available as Underlying data40–42.
The results show that adding activated charcoal to the diet of C. ignobilis significantly affected favoela gastrica and intestinal villi biometrics. According to Pirarat et al.19, activated charcoal plays a significant role stimulating the development of epithelial cells of the digestive organs. Activated charcoal in the diet functions as a decontaminating agent to eliminate pathogenic organisms and toxic compounds, such as mycotoxins20. Hence, a longer foveola gastrica and larger intestinal villi were able to provide more nutrients to be absorbed due to a larger surface area of digestive organs43. Optimal development of the alimentary tract was recorded in giant trevally juveniles fed the experimental diet containing rice husk charcoal. This was presumably due to the high hemicellulose, cellulose, and lignin contents in the rice husk charcoal. A previous report indicated that rice husk charcoal contains 29.3% hemicellulose, 34.4% cellulose, and 19.2% lignin44, while mangrove wood charcoal has 30% hemicellulose, 36% cellulose, and 28% lignin45, coconut shell charcoal has 19.27% hemicellulose, 33.61% cellulose, and 36.51% lignin46, and kernel palm shell charcoal has 26.27% cellulose, 12.61% hemicellulose, and 42.96% lignin47. Maria and Banu48 and Jamilatun et al.49 reported that the concentration and quality of charcoal depend on the composition of hemicellulose, cellulose, and lignin. The quality of the activated charcoal is higher when these three components increase. According to Jasman50, rice husk contains 85–95% activated charcoal, while mangrove wood has 76% activated charcoal51, kernel palm shell 65% activated charcoal47, and coconut shell has 60% activated charcoal46.
The microscopic observations showed that the intestinal villi of the fish fed the diet with activated rice husk charcoal had a more pointed shape compared to other treatments, in which the villi tended to be round and blunt. According to Guo et al.52, blunt or rounded villi probably occur due to inflammation in the intestinal mucosa, which is characterized by infiltration of neutrophils into the lamina propria. An increase of intestinal villus size is related to nutrient absorption capacity. According to Nafis et al.53, long mucosal folds increase nutrient absorption and reduce food flow movement due to reduced peristaltic contractions, which provides sufficient time to optimally absorb nutrients. The increase in intestinal villi size is strongly related to the activities of digestive enzymes, such as lactase, sucrase, alkaline phosphatase, and disaccharidase54–57.
The morphology of the intestinal villi of fish fed a diet without activated charcoal was wider and shorter than that of fish fed the diets with activated charcoal. This was probably due to impaired intestinal mucosal integrity, causing interference in nutrient absorption. According to Choct58, shortening of the intestinal villi is related to the accumulation of intestinal pathogenic bacteria, resulting in increased susceptibility to infection in the intestinal mucosal layer. This causes the digestive organs to form more secretory cells than absorbent cells, which reduces nutrient uptake59,60. The active charcoal likely acts as an adsorbent of metabolic pathogens in the intestine in the form of endotoxins and ammonia, therefore, it was able to improve intestinal function61.
The application of activated charcoal in the diet significantly affected the length and width of the foveola gastrica and intestinal villi of giant trevally, C. ignobilis. The optimal biometrics of the foveola gastrica and intestinal villi were observed in fish fed the experimental diet with activated rice husk charcoal.
Figshare: Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoal. https://doi.org/10.6084/m9.figshare.12203525.v240.
This project contains the following underlying data:
DATA BIOMETRIC GUT OF GIANT TREVALLY Caranx ignobilis_Edited (XLSX). (Raw biometric data for the foveola gastrica of all fish examined in this study.)
DATA BIOMETRIC OF INTESTINE OF GIANT TREVALLY Caranx ignobilis_edited (XLSX). (Raw biometric data for the intestinal villi of all fish examined in this study.)
Figshare: Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoal. https://doi.org/10.6084/m9.figshare.12301124.v241.
This project contains uncropped, unprocessed images of the intestinal villi of giant trevally.
Figshare: Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoaltem. https://doi.org/10.6084/m9.figshare.12269606.v242.
This project contains uncropped, unprocessed images of the foveola gastrica of the giant trevally.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank the Kemenristekdikti for supporting this study. All staff at the Center for Brackish Water Aquaculture in Ujung Batee who assisted with this study are acknowledged. Special thanks to Mr. Boihaqi and Maisyarah Rita for their assistance during the study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Aquaculture, Aquaponics, fish nutrition
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Aquaculture
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Aquatic Chemistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 13 Oct 20 |
read | |
|
Version 1 26 May 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)