Keywords
Evidence-based Medicine, Randomized controlled Trials, Research design, Informed consent, Clinical trials, Audiovisual aids, mechanical ventilation, cycle ergometry, Studies within a trial
This article is included in the Studies Within A Trial (SWAT) collection.
Evidence-based Medicine, Randomized controlled Trials, Research design, Informed consent, Clinical trials, Audiovisual aids, mechanical ventilation, cycle ergometry, Studies within a trial
In-bed cycling is a novel critical care rehabilitation intervention that could improve patients’ physical function1. In-bed cycling is not commonly used in the intensive care unit (ICU)2 and is likely unfamiliar to many patients and their substitute decision makers (SDMs). A video demonstrating the use of the cycle in addition to verbal informed consent may improve their satisfaction and comfort with the consent process. The purpose of this study within a trial (SWAT) was to determine the effect of video-augmented versus standard informed consent on patient and SDM satisfaction and comfort.
The study protocol is available as Extended data3.
We included a convenience sample of consent encounters in TryCYCLE (NCT01885442), a 33-patient, single-centered, safety and feasibility study of in-bed cycling in critically ill patients4. The TryCYCLE study required a priori informed consent. Patients and SDMs who were approached for in-person informed consent for TryCYCLE in our mixed medical-surgical ICU (St Joseph’s Healthcare ICU) in Hamilton, ON were eligible for this substudy. We excluded encounters where initial consent was received by phone, as respondents could not view the video as part of their initial deliberations.
Using concealed allocation with variable undisclosed block size, we randomized consent encounters to receive augmented video consent in addition to verbal consent discussion (Video) or verbal consent discussion only (Standard) in a 1:1 ratio. To maintain allocation concealment, we used third-party assignment. An individual not otherwise involved in the study (MD) prepared a computer-generated randomization sequence and provided the group assignment immediately prior to each consent encounter.
The research coordinator used a tablet computer to show and describe how an ICU patient would bike in the TryCYCLE study. The 2-minute video included footage of a model on the bike and an ICU patient biking while mechanically ventilated. The research coordinator also provided a verbal description of the research study, which was not scripted to allow for spontaneous exchange. The research coordinator and patient and/or SDM had the consent encounter guided by the usual principles of informed consent for ICU studies previously published5. The video did not include sound.
Comparison: Verbal description only.
Our primary outcomes were patient and/or SDM satisfaction and comfort with the consent encounter. Secondary outcomes included patient and/or SDM perception of video helpfulness, and research coordinator assessments of satisfaction with the consent process, their own comfort with the consent encounter, patient and/or SDM study comprehension, and the number and perceived difficulty of questions asked by patient and/or SDM. Respondents assessed outcomes after the consent encounter using 7-point Likert-type scales, with higher scores representing more favorable ratings (see Extended data3). We invited patients and/or SDMs to return the 1-page, self-administered questionnaire within 24 hours in a pre-addressed, sealable envelope. The research coordinator documented consent outcome and duration of the encounter.
Patients and SDMs were not aware of the comparison study at the time of the consent encounter. Both groups received the same consent forms for the TryCYCLE study3. The analyst was blinded to treatment group.
We did not calculate a sample size a priori for this SWAT. We reported continuous variables as mean (standard deviation [SD]) and categorical values as count (percent). We used Student’s t-tests to compare the results of the two groups, Pearson’s Chi Square for categorical comparisons, and p=0.05 as the criterion for statistical significance. We conducted all analyses with SPSS version 25.0 (IBM, Armonk, New York).
We randomized 14 of the 37 consent encounters for TryCYCLE between October 2013 and August 2014: 6 to Video and 8 to Standard (Figure 1). Table 1 summarizes the characteristics of the convenience sample in this study. We received 10 completed questionnaires (5 from each group), which contributed to estimates of effect. We report all comparisons as Video vs. Standard. Patient and/or SDM rating of satisfaction was not different between groups, the mean [SD] was 6.8 [0.5] vs. 7.0 [0] (p=0.37). Patient and/or SDM rating of comfort was the same for both groups (7.0 [0], p>0.99). Patient and/or SDM rating of video helpfulness was 6.6 [0.54]. All 14 (100%) patients and/or SDMs provided consent to participate in the TryCYCLE study (the overall consent rate for TryCYCLE was 91.9%)4.
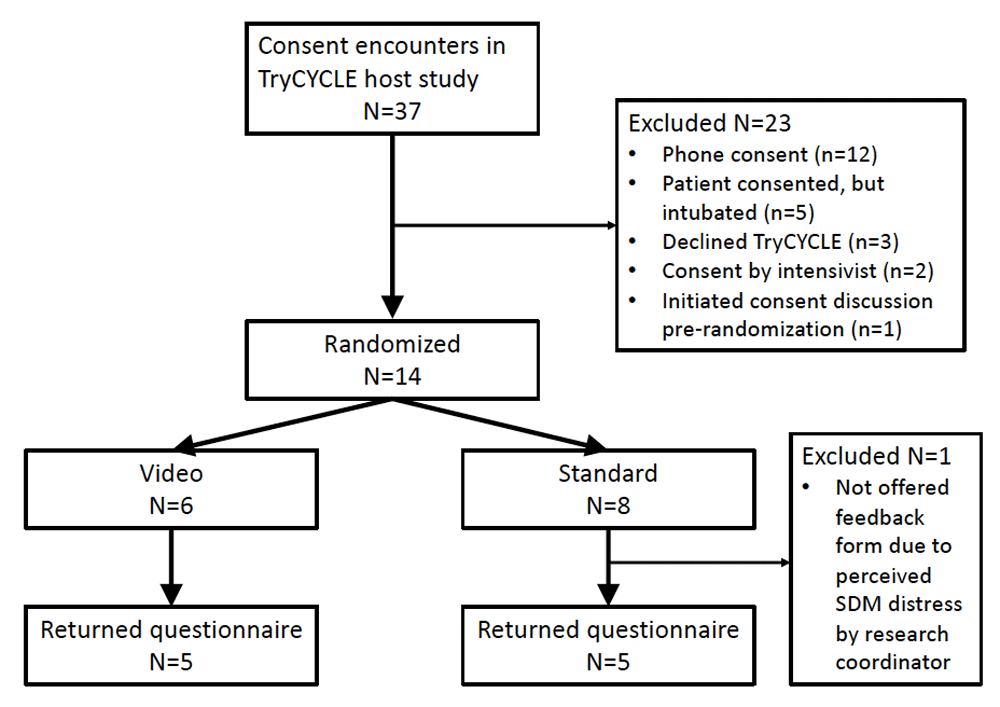
Of the 23 non-randomized patients who had consent encounters, 12 occurred by phone, 5 were in-person by intubated patients who were unable to complete the feedback form, 3 declined participation in the host study and these consent encounters occurred without the option for enrolment in the SWAT, 2 in-person consents were obtained by a consultant intensivist, and in 1 instance we already initiated the consent discussion before consideration of randomization in this trial. Post-randomization, 1 person was not offered the feedback form due to perceived emotional distress by the research coordinator.
SDM= substitute decision maker.
Of the 14 consent encounters rated by the research coordinator, satisfaction (7.0 [0] vs. 6.9 [0.35], p=0.41) and comfort (6.7 [0.52] vs. 6.9 [0.35], p=0.39) were similar. There was no difference in perceived patient and/or SDM understanding of the research study (6.67 [0.51] vs. 7.0 [0] vs., p=0.11), number of questions asked (1-5 questions: 6 (75.0%) vs. 5 (83.3%), p=0.66), nor perceived difficulty of clarifying questions (2.67 [1.86] vs. 1.86 [1.4], p=0.40). The encounter duration was not different (18.3[14.7] vs. 13.3[3.7] minutes, p=0.20).
In this small randomized study of video-augmented versus standard consent for in-bed cycling in the ICU, there was no difference in satisfaction or comfort between groups. Our results are similar to previous studies included in a systematic review of audiovisual plus standard versus standard informed consent, identifying no difference in consent rate between groups6. Given the universally high ratings for satisfaction and comfort by consent recipients, we hypothesize that video-augmented consent could be useful for describing more complex interventions or facilitating conversations when research centres or coordinators are new to conducting clinical trials. Involving patient-family advisory groups may help guide use and content of video adjuncts for future consent encounters.
Our study also contributes to global efforts to study efficiencies in trial conduct through SWATs7. It directly addresses Priority 4 (What are the best approaches for designing and delivering information to members of the public who are invited to take part in a randomized trial) of the top 10 trial recruitment issues informed by stakeholders, including members of the public, front line clinical and research staff, trial principal investigators, and funders8.
Our study has limitations, principally the small sample size and significant expertise in consent discussions from a single research coordinator with 20 years of experience in critical care studies. We did not measure consent recipient knowledge. Our study also has several strengths. Patients and/or SDMs were blinded to the Video vs Standard comparison study. We evaluated practical aspects of the consent process, focusing on outcomes we anticipate are important to patients and SDMs. We addressed gaps in previous research by measuring SDM and clinical researcher satisfaction, and time to administer the video-augmented consent6.
Overall, patient, SDM, and research coordinator satisfaction and comfort with consent was very high for both the video-augmented and standard approaches. Further research, including use of videos to portray different study interventions, is needed, including analysis of patient and/or SDM satisfaction, comfort, comprehension, and consent rates.
Since the intervention video includes an identifiable critically ill patient, and permission to publish this was not obtained from the patient, patient’s family or the research ethics board, this cannot be shared.
Open Science Framework: Video-augmented vs. standard consent in an early ICU cycling feasibility trial: a randomized embedded recruitment trial, https://doi.org/10.17605/OSF.IO/4SPGA3
This project contains the following underlying data:
Open Science Framework: Video-augmented vs. standard consent in an early ICU cycling feasibility trial: a randomized embedded recruitment trial, https://doi.org/10.17605/OSF.IO/4SPGA3
This project contains the following extended data:
Open Science Framework: CONSORT checklist for ‘Video-Augmented vs Standard Consent in an Early ICU Cycling Feasibility Trial: A Randomized Embedded Recruitment Trial’, https://doi.org/10.17605/OSF.IO/4SPGA3
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Intensive care medicine, clinical research
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Interest in SWATS and their conduct as well as being the Director of Trials Unit
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 24 Jan 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)