Keywords
diabetic ulcer, ozone, virgin coconut oil, full-thickness wound, delayed wound healing, HSP90α, VEGF-A, EGF, bFGF, CD34
diabetic ulcer, ozone, virgin coconut oil, full-thickness wound, delayed wound healing, HSP90α, VEGF-A, EGF, bFGF, CD34
1. Revised grammar
2. Shortened the introduction
3. Chart has been replaced with bar chart version
4. Several other fixes to the article grammar and formatting for better clarity
See the authors' detailed response to the review by Rekha Raghuveer Shenoy
See the authors' detailed response to the review by Gamal Badr
Diabetes is a non-communicable disease that has become a worldwide burden. In 2014, the number of people with diabetes in the world reached 8.5% of the total world population. According to a WHO report, the prevalence of diabetes has kept increasing steadily for the last three decades, especially in low-income and middle-income countries such as Indonesia1. In 2013, the prevalence of diabetes mellitus among productive-age citizens of Indonesia was 4.6%2. In line with the WHO report, the International Diabetes Federation found that in 2017, the prevalence of diabetes mellitus among adults in Indonesia had increased to 6.7%.
Diabetes is a risk factor and cause of several other diseases, including blindness, kidney failure, diabetic neuropathy, diabetic vasculopathy, and diabetic ulcers. Of those diabetic patients, 15% of them will develop a diabetic ulcer. If the diabetic ulcer is not managed properly, it can develop into gangrene, thus increasing the likelihood of amputation. 12–24% of patients who have diabetic ulcers end up requiring amputation3–6.
Diabetic ulcers are a serious complication in patients with diabetes7. A diabetic ulcer is defined as the presence of a full-thickness lesion located distally relative to the ankle. Diabetic ulcers are caused by a combination of several risk factors, including neuropathy, peripheral arterial occlusive disease, and previous ulcerations7,8. Of all diabetic patients worldwide, 6.3% are affected by diabetic ulcers9. Diabetic ulcers are limb-threatening conditions that can potentially result in the amputation of a limb1,7.
The management of diabetic ulcers needs a holistic approach, taking into consideration systemic conditions, complications of neuropathy or atherosclerosis, and classification of injuries. Treatment of diabetic ulcer wounds involves three stages, consisting of cleansing, debridement, and dressing. Besides, pressure control and infection control are also needed. Ulcers can become an area of bacterial growth that must be treated with antibiotics according to the results of bacterial culture13–15.
Virgin coconut oil (VCO) is a product of coconut that is produced by processing fresh coconuts at a low temperature. This processing method keeps important substances in the coconut oil intact and ensures they do not degrade. VCO mainly contains 90% saturated fatty acids and 10% unsaturated fatty acids. VCO contains antioxidants such as tocopherol and beta carotenes, and has properties to enhance moisturization of the skin16. VCO has been shown to increase wound contraction rate in chronic wounds that exhibited delayed wound healing, and have the potential to increase wound healing biomarker levels in a full-thickness wound mouse model17,18.
Ozone is an inorganic triatomic molecule composed of three oxygen atoms and is highly soluble19,20. Ozone is known to have a role in protecting the ecological balance of the earth and can interact at a basic level with industrial pollutants. Ozone also has a unique biological ability that has the potential to be used in the medical field. Medical uses of ozone use ozone dissolved in water (ozonated water), ozonated oils, and ozone in gas form21. Previous studies found that ozone acts as an agent that helps ulcer wound healing in patients with diabetes mellitus. Ozone interactions with skin tissue can: cause inactivation of bacteria, viruses and fungi; stimulate antioxidant production; reduce blood and plasma viscosity; increase erythrocyte membrane fluidity; loosen tissue; stimulate hemoglobin activity and increase oxygen absorption and release; improve blood circulation to tissues; induce tissue collagen formation; activate the formation of granulation tissue; accelerate epithelialization; and increase the phagocytic activity and activation of fibroblasts. Ozone also supports the treatment of diabetes mellitus through decreasing blood sugar levels and increasing oxygen supply into the tissues24–26.
Heat shock protein 90 (HSP90) is a protein chaperone released and upregulated from pancreatic beta cells under inflammatory conditions27. HSP90 is a member of the heat shock protein superfamily and itself consists of HSP90α and HSP90β28. HSP90 is excreted when normal cells are injured and heat shock proteins, especially HSP90α, are essential for the early phase of wound healing because they promote keratinocyte differentiation, re-epithelialization, dermal cell recruitment, and also protect tissues from further injury29–31. Vascular endothelial growth factor (VEGF) has an important role in the coordination of wound healing32. VEGF-A exhibited mis-regulation in diseases with microvascular disorders33. Diabetes causes reduced VEGF signaling, alongside impaired angiogenesis and decreased collateral blood vessel formation34. Besides causing microvascular and macrovascular disorders, this impaired angiogenesis also has a significant role in the pathogenesis of diabetic wound healing35. Increased VEGF-A is beneficial in diabetic wound healing by increased angiogenesis, vessel diameter, re-epithelialization and wound closure36. Epidermal growth factor (EGF) plays an important role in wound healing through stimulation, proliferation and migration of several types of cells, such as keratinocytes, epithelial cells, endothelial cells and fibroblasts, thus facilitating dermal regeneration37,38. EGF is synthesized in the pancreas, and its levels are decreased in diabetic animal models38. Increased EGF levels might have several benefits for wound healing in diabetic wounds and ulcers by increasing granulation tissue formation in diabetic wounds39–41. Basic fibroblast growth factor (bFGF) aids in wound healing42. bFGF influences chemotaxis, cell differentiation, cell proliferation, and tissue regeneration, and was found to increase epithelial wound healing in an early study43,44. CD34+ is a biomarker that can be used to predict the healing of diabetic wounds. CD34+ levels are decreased in subjects with diabetes compared to subjects with normal glucose tolerance, and lower levels of CD34+ are also correlated with a significantly higher risk of diabetes46,47. Levels of sphingosylphosphorylcholine (SPC) in the bloodstream can be used to predict the healing of diabetic neuropathic wounds, which might show its significance in improving the healing of diabetic wounds48.
Currently, there are limited studies of ozone treatment in animal models of diabetic ulcers and a lack of clinical trials concerning the efficacy of ozone treatment for ulcers in patients with diabetes mellitus, especially in Indonesia. We used VCO as the ozone carrier liquid in this study. This study aims to evaluate the effect of topical ozonated VCO using a diabetic wound rat model, assessed by macroscopic wound improvement and immunohistochemical staining parameters.
This study is an experimental study with a post-test control design. This research was approved and declared ethically feasible by the Ethics Commission of the Public Health Faculty, Diponegoro University Semarang with ethical clearance number 078/EC/FKM/2018. All efforts were made to ameliorate harm to animals by administering anesthesia to all of the study animals before full-thickness wound model creation, keeping the animals in a well-maintained cage, and ensuring graceful termination of animals before we took tissue samples for a histopathology examination.
The subjects of this study were male adult laboratory rat (Wistar strain). The rat were aged three months with a mean weight of 250 ± 50 grams. We obtained the rat from a local laboratory-grade rat breeder, with a pure-breeding status. The rat included in this study fulfilled the inclusion criteria for healthy conditions (active movement), and exhibited no signs that met our exclusion criteria: behavioral changes (activities seemed weak and lazy). The choice of rat strain and size is related to the wound model creation; the large size of Wistar rat enables us to have an ample area on the rat back to create the full-thickness wound model. Rat were kept in stainless-steel laboratory-grade mouse cages at a constant room temperature of 28.0±2.0°C, which was monitored with a digital thermometer. The rat were kept in our laboratory mouse room in cages with wood shavings as the bedding material, which was changed once a week. Each cage was inhabited by two to three rat according to the cage size. We did not mix rat from different groups in one cage. Fluorescent lighting was turned on for 12 hours per day between 9.00 AM to 9.00 PM, with adequate ad libitum food supply (BioFeed pellets, manufactured by Karunia Kasih Abadi, Klaten, Indonesia) and water supply (clean drinkable water). We routinely assessed the clinical and behavioral status of the rat every day during routine checks to prevent any clinical or behavioral changes in the laboratory rat. No rat were found dead, having decreased appetite/growth, or exhibited behavioral changes during the study period.
In the diabetic group, we induced type 1 diabetes in the rat by injecting a single dose of 40mg/kg body weight streptozotocin, dissolved in a buffer solution of 50 mM citrate with pH of 4.5 to obtain a final concentration of 40 mg/ml. We assessed the blood glucose level before streptozotocin injection as a baseline value and repeated 10 days after streptozotocin injection to determine whether the diabetic induction was successful or not. The measurements of blood glucose level was done using glucose oxidase phenol 4-aminoantipyrine (GOD-PAP) obtained from Diagnostic Systems International. The diagnosis of type 1 diabetes was made if blood glucose levels reached more than 200 mg/dl (11.1 mmol/liter) 10 days after streptozotocin injection, or if blood glucose levels reached twice the baseline blood sugar noted at the time of injection.
The manufacture and testing of ozonated VCO were carried out at the Plasma Research Center (PRC), Diponegoro University, Indonesia. The tools used for making ozonated oil were ozone generators (ozone generator manufactured in-house by Plasma Research Center, Diponegoro University) and magnetic stirrers. The ozone outlet is connected to an anti-oxidation hose with a diffuser, which served to increase the effectiveness of ozone absorption in the oil. Magnetic stirrers were used to facilitate the ozone dissolving process into the oil. The oil used in this study was VCO. Ozone was dissolved into VCO with a volume of 100 cc in each cycle and an oxygen flow rate of 0.1 liters/minute with an ozone concentration of 3360 ppm. We varied the duration of the ozonation process to create several concentrations of ozonated oil. The duration of ozonation was 90 minutes, 7 hours, and 14 hours.
The full-thickness wound model, treatment application, and observation were done simultaneously on all study groups; however, the diabetic induction was conducted 10 days earlier before the wound model creation to make sure all rat were ready to undergo wound model creation simultaneously in their final state (diabetic or non-diabetic according to the group). The research process began by inducing the diabetic ulcers in the Wistar rat in the diabetic group. We soaked cotton with 0.05ml of chloroform and put the cotton into a glass beaker containing a mouse. This allowed us to carry out aerosol administration of anesthesia in a similar manner of anesthesia aerosol gas administration in humans who underwent surgery, which we applied for two minutes. The administration of anesthesia was carried out directly before we excised the wounds, at our laboratory mouse surgery table, which was cleaned and sanitized before the process began to minimize the risk of contamination from the surgical field or surgical apparatus. After hypoesthesia was achieved (measured by loss of muscular tone, loss of righting and palpebral reflexes, decreased rate of respiration, and loss of response to painful stimulation), the hair in the region of the spine at the top of the back (thoracolumbar area) was shaved49. Then we made four excisions, each with a diameter of 6 mm, separated by the median line. The full-thickness skin excision was done using punch biopsy with a depth of 1 mm.
A total of 60 rat were used in this research. The sample was then divided into six groups randomly (achieved by simple randomization), where each group consisted of 10 rat. The minimum sample size for this study was obtained according to the Frederer sample size calculation formula: (t-1) (n-1) ≥ 15; where t = number of groups and n = number of replications/number of minimum samples per group. There are six groups in this study; therefore, the formula was (6-1) (n-1) ≥ 15, which results in 5n ≥ 20. We added more rat (twice the sample amount) due to the need to obtain three rat every histopathology examination, which was done three times during the study (explained in the next section). These groups were: the negative control group (C-), a control group of rat with negative diabetes mellitus status that received conventional treatment such as washing the wound with normal saline and gentamycin antibiotic ointment (Kimia Farma pharmaceutical industry, Indonesia); the positive control group (C+), a control group with positive diabetes mellitus status that received similar conventional treatment to the negative control group, such as washing the wound with normal saline and gentamycin antibiotic ointment (Kimia Farma pharmaceutical industry, Indonesia); the first treatment group (P1), which received normal saline wound wash and non-ozonated VCO treatment; the second treatment group (P2), which received normal saline wound wash and 90-minute ozonated VCO oil therapy; the third treatment group (P3), which received normal saline wound wash and 7-hours ozonated VCO oil therapy; and the fourth treatment group (P4), which received normal saline wound wash and 14-hours ozonated VCO oil therapy.
We applied the oils (ozonated VCO or regular VCO according to the group) to the wound once a day in the morning (approximately 8 a.m or 9 a.m) for fourteen consecutive days, thinly covering the entire wound surface with each treatment. The rat were first taken out of the cage, received the treatment at our mouse surgical table (which was cleaned and sanitized beforehand), and then returned back to the cage. The wound was left open and not covered with any kind of covering. For the macroscopic wound contraction measurement, we measured the wound contraction length using a digital measurement caliper (0,01 mm measurement scale, 150mm maximum length) on the first day, third day, fifth day, seventh day and fourteenth day before we applied the ozonated VCO each day, and recorded it in a follow-up table for further analysis.
For the immunohistochemistry staining, we analyzed the wound three times during the study period: on the first day, on the third day, and the seventh day. We sacrificed three rat each period according to the standard operational procedure for immunohistochemistry staining50. The anesthesia was administered by aerosol chloroform, similar to the method we used earlier when we created the wound model. We did not use injectable anesthesia due to our concern that the injectable anesthesia might disturb the histopathological appearance of the tissues. The rat were first anesthetized using ether gas before we took samples from the wound margin. Afterwards, we fixed the slices using 10% neutral buffered formalin for 24 hours, embedded it into a paraffin block and thinly sliced it. We then measured the immunohistochemistry levels using specific staining for each of the biomarkers. The immunohistochemistry interpretation was done by pathology experts and was done blindly to make sure there was no subjectivity bias. The inclusion of each mouse in each immunohistochemistry period was randomized to prevent any bias. The remaining rat were terminated according to animal ethics by cervical dislocation.
During the research, none of the 60 rat dropped out or died before the research process ended and there were no observed adverse effects in the animals used in this study.
The measurements of wound length on day 1 to 14 (Table 1) as an indicator of wound healing shows a better wound healing process for the VCO with a longer duration of ozone flow. The reduction of wound length is directly proportional to the duration of ozone flow. The greatest to smallest wound length reduction is found in the VCO with ozone flow for 14 hours group (P4), followed by the 7 hours ozonated VCO group (P3), 90 minutes ozonated VCO group (P2), non-ozonated VCO group (P1), and the diabetic wound without VCO group (C-). The P4 group, which received VCO with ozone flow for 14 hours shows the greatest reduction in wound length (16837.10 µm)51. The smallest reduction in wound length was found in the C+ group (6370.77 µm), as expected. The results can be seen in Table 1 and Figure 1.
C-, negative control group with negative diabetic status; C+, positive control group with positive diabetic status; P1, non-ozonated VCO therapeutic group; P2, 90-minute ozonated VCO therapeutic group; P3, 7-hours ozonated VCO therapeutic group; P4, 14-hours ozonated VCO therapeutic group; VCO, virgin coconut oil.
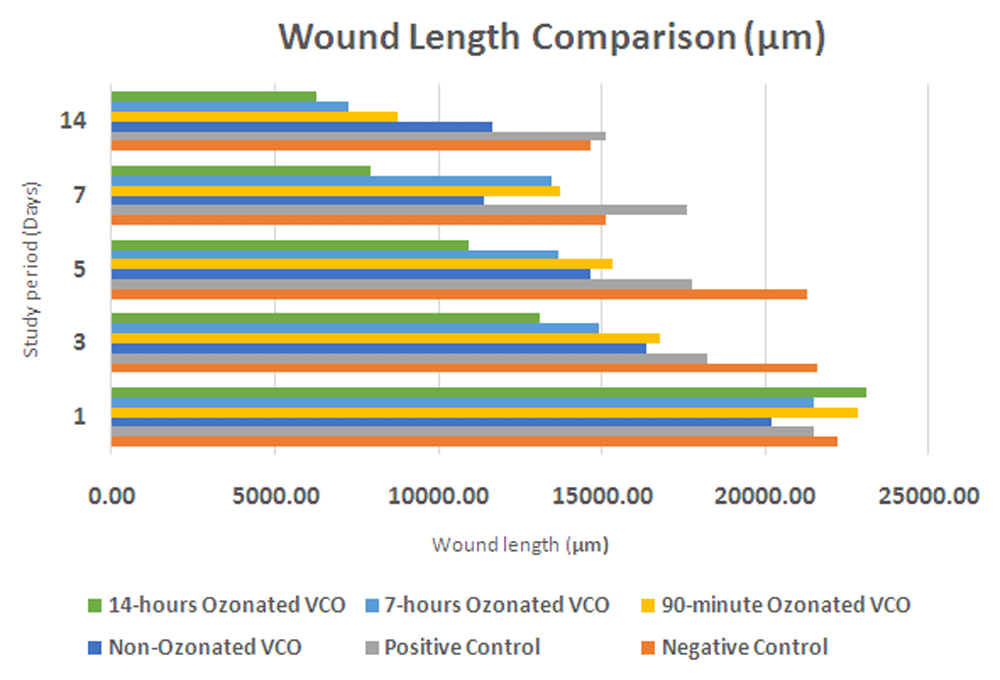
VCO, virgin coconut oil.
Figure 2 shows the macroscopic wound morphology comparison of wound healing between the study groups. As shown in the picture, the wounds in the treatment groups healed much faster compared to the positive control group without any treatment. The fastest wound healing was found among the P4 group, which had almost closed completely on the 14th day, where at the same time the wound on the positive control group had still barely healed. The P3 group had a similar wound healing result, although the wound length is still greater than that of the P4 group, signifying that the P4 group achieved the best wound healing result compared to all other therapeutic groups. Therefore, these results suggest that topical application of ozonated VCO can improve wound healing in diabetic rat, as shown by the improved wound contraction length compared to the diabetic positive control group without any treatment.
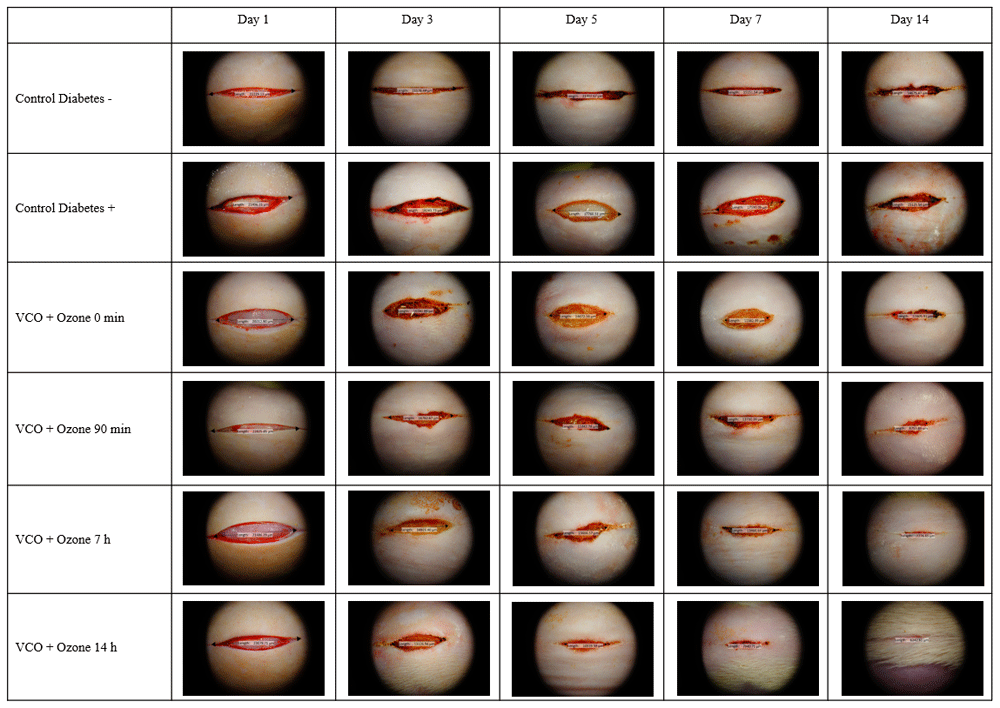
The wound for the 14-hours ozonated VCO group healed faster and better compared to the control group.
VCO, virgin coconut oil.
Descriptive measurements of HSP90Α levels are shown in Table 2. The lowest HSP90Α levels were obtained in the C+ group and the highest HSP90Α levels were obtained in the P4 group throughout the measurement period. The histopathological images of HSP90a-stained cells are shown in Figure 3. One-way ANOVA showed a significant difference for all three measurement days. For the first day measurement, both the P3 group and P4 group exhibited significantly higher HSP90Α levels compared to the C+ group. No group showed significantly higher HSP90A levels as compared to the C- group for the first-day measurement. For the third day measurement, all therapeutic groups exhibited significantly higher HSP90Α levels compared to the C+ group, with P2, P3 and P4 groups showing significantly higher HSP90Α levels compared to the C- group. Results for the seventh-day measurement were similar to the third-day measurement, where all therapeutic groups exhibited significantly higher HSP90Α levels than to the C+ group, with P3 and P4 groups showing significantly higher HSP90Α levels compared to the C- group.
C-, negative control group with negative diabetic status; C+, positive control group with positive diabetic status; P1, non-ozonated VCO therapeutic group; P2, 90-minute ozonated VCO therapeutic group; P3, 7-hours ozonated VCO therapeutic group; P4, 14-hours ozonated VCO therapeutic group; VCO, virgin coconut oil.
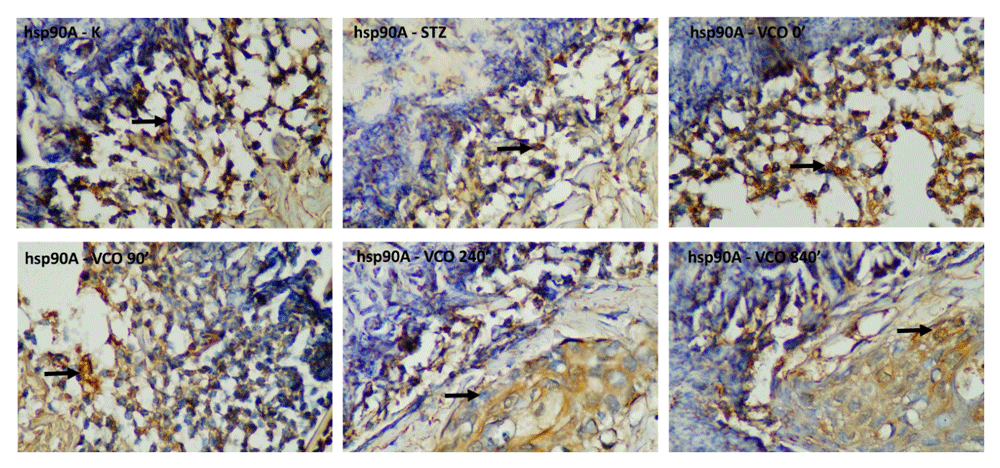
Top row, from left to right: negative control group with negative diabetic status; positive control group with positive diabetic status; non-ozonated VCO therapeutic group. Bottom row, from left to right: 90-minute ozonated VCO therapeutic group; 7-hour ozonated VCO therapeutic group; 14-hour ozonated VCO therapeutic group. The size bar indicates 20 µm. VCO, virgin coconut oil.
Descriptive measurements of VEGF-A levels are shown in Table 3. The lowest VEGF-A levels were obtained in the C+ group and the highest VEGF-A levels were obtained in the P4 group throughout the measurement period. The histopathological images of VEGF-stained cells are shown in Figure 4. One-way ANOVA showed a significant difference for all three measurement days. For the first day measurement, significantly higher VEGF-A levels were found for P2, P3 and P4 groups as compared to the C+ group, with P3 and P4 groups showing significantly higher VEGF-A levels compared to the C- group. For the third-day measurement, P2, P3 and P4 groups exhibited significantly higher VEGF-A levels compared to the C+ group, with only the P4 group showing significantly higher VEGF-A levels compared to the C- group. Results for the seventh-day measurement shows similar results to the first- and third-day measurements, where P2, P3 and P4 groups exhibited significantly higher VEGF-A levels compared to both C+ and C- groups.
C-, negative control group with negative diabetic status; C+, positive control group with positive diabetic status; P1, non-ozonated VCO therapeutic group; P2, 90-minute ozonated VCO therapeutic group; P3, 7-hours ozonated VCO therapeutic group; P4, 14-hours ozonated VCO therapeutic group; VCO, virgin coconut oil.
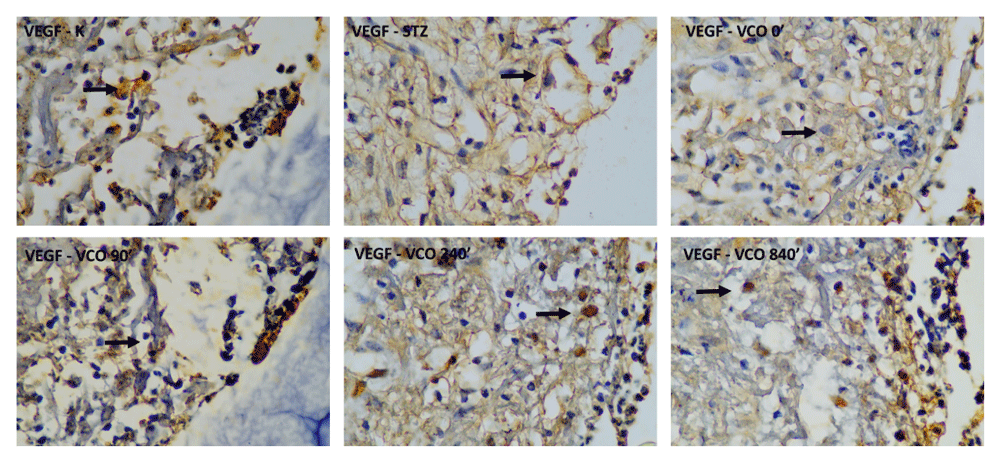
Top row, from left to right: negative control group with negative diabetic status; positive control group with positive diabetic status; non-ozonated VCO therapeutic group. Bottom row, from left to right: 90-minute ozonated VCO therapeutic group; 7-hours ozonated VCO therapeutic group; 14-hours ozonated VCO therapeutic group. Size bar indicates 20 µm.
VCO, virgin coconut oil.
Descriptive measurements of EGF levels are shown in Table 4. The lowest EGF levels were obtained in the C+ group and the highest EGF levels were obtained in the P4 group throughout the measurement period. The histopathological images of EGF-stained cells are shown in Figure 5. One-way ANOVA showed a significant difference for all three measurement days. For the first day measurement, significantly higher EGF levels were found for P2, P3 and P4 groups compared to the C+ group, with no group showing significantly higher EGF levels compared to the C- group. For the third day measurement, all therapeutic groups exhibited significantly higher EGF levels compared to the C+ group, with the P3 group showing significantly higher EGF levels compared to the C- group. Results for the seventh-day measurement show that all therapeutic groups exhibited significantly higher EGF levels compared to the C+ group, with P2, P3 and P4 groups showing higher EGF levels compared to C- group.
C-, negative control group with negative diabetic status; C+, positive control group with positive diabetic status; P1, non-ozonated VCO therapeutic group; P2, 90-minute ozonated VCO therapeutic group; P3, 7-hours ozonated VCO therapeutic group; P4, 14-hours ozonated VCO therapeutic group; VCO, virgin coconut oil.
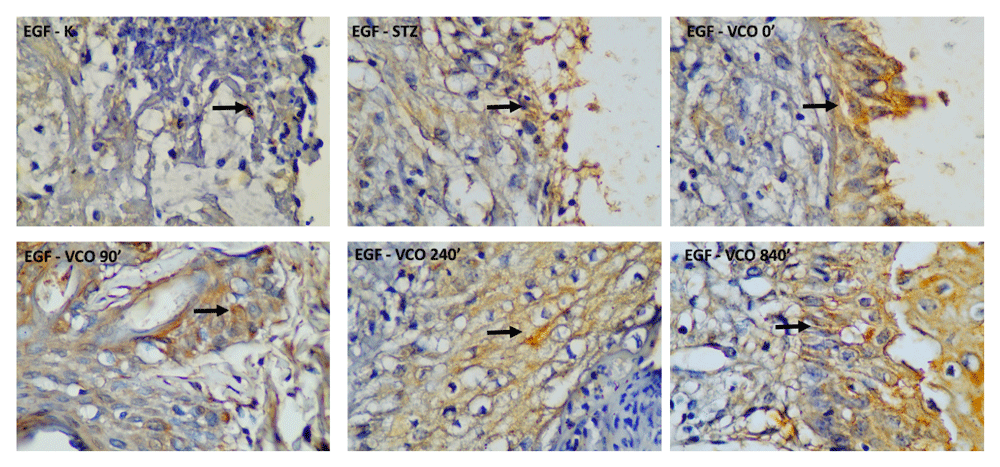
Top row, from left to right: negative control group with negative diabetic status; positive control group with positive diabetic status; non-ozonated VCO therapeutic group. Bottom row, from left to right: 90-minute ozonated VCO therapeutic group; 7-hour ozonated VCO therapeutic group; 14-hour ozonated VCO therapeutic group. Size bar indicates 20 µm. VCO, virgin coconut oil.
Descriptive measurements of bFGF levels are shown in Table 5. The lowest bFGF levels were obtained in the C+ group and the highest bFGF levels were obtained in the P4 group throughout the measurement period. The histopathological images of bFGF-stained cells are shown in Figure 6. One-way ANOVA showed a significant difference for all three measurement days. For the first day measurement, significantly higher bFGF levels were found for P2, P3 and P4 groups compared to the C+ group, with only P4 showing significantly higher bFGF levels compared to the C- group. For the third day measurement, all therapeutic groups exhibited significantly higher bFGF levels compared to the C+ group, with P2, P3, and P4 groups showing significantly higher bFGF levels compared to the C- group. Similar to the third day, results for the seventh-day measurement show that all therapeutic groups exhibited significantly higher bFGF levels compared to the C+ group, with P2, P3 and P4 groups showing higher bFGF levels compared to the C- group.
C-, negative control group with negative diabetic status; C+, positive control group with positive diabetic status; P1, non-ozonated VCO therapeutic group; P2, 90-minute ozonated VCO therapeutic group; P3, 7-hour ozonated VCO therapeutic group; P4, 14-hour ozonated VCO therapeutic group; VCO, virgin coconut oil.
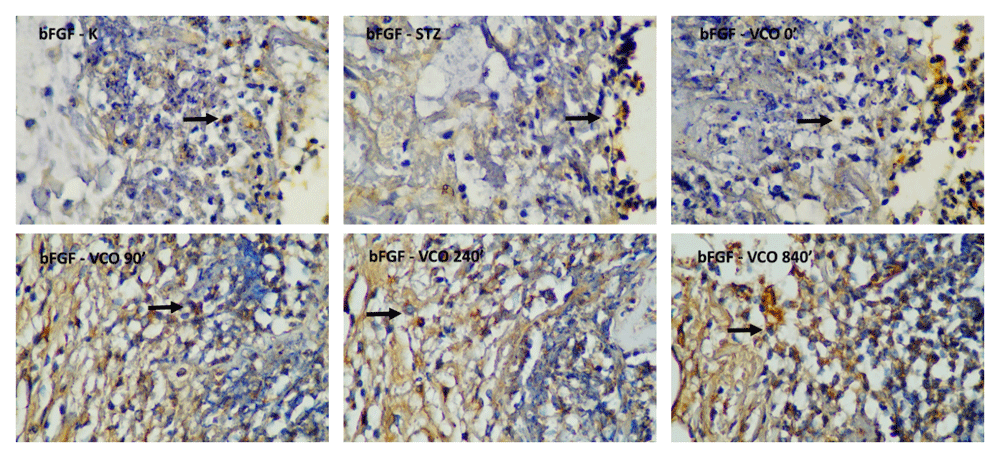
Top row, from left to right: negative control group with negative diabetic status; positive control group with positive diabetic status; non-ozonated VCO therapeutic group. Bottom row, from left to right: 90-minute ozonated VCO therapeutic group; 7-hour ozonated VCO therapeutic group; 14-hour ozonated VCO therapeutic group. The size bar indicates 20 µm. VCO, virgin coconut oil.
Descriptive measurements of CD34 levels are shown in Table 6. The lowest CD34 levels were obtained in the C+ group and the highest CD34 levels were obtained in the P4 group throughout the measurement period. The histopathological images of CD34-stained cells are shown in Figure 7. One-way ANOVA showed a significant difference for all three measurement days. For the first day measurement, significantly higher CD34 levels were only found for the P4 group compared to both C+ and C- groups. For the third day measurement, all therapeutic groups exhibited significantly higher CD34 levels compared to the C+ group, with P2, P3 and P4 groups showing significantly higher CD34 levels compared to the C- group. Similar to the third day, results for the seventh-day measurement show that all therapeutic groups exhibited significantly higher CD34 levels compared to the C+ group, with only the P4 group showing higher CD34 levels compared to the C- group. Therefore, the P4 group most consistently had higher levels of all growth factors and biomarkers measured in this study.
C-, negative control group with negative diabetic status; C+, positive control group with positive diabetic status; P1, non-ozonated VCO therapeutic group; P2, 90-minute ozonated VCO therapeutic group; P3, 7-hour ozonated VCO therapeutic group; P4, 14-hours ozonated VCO therapeutic group; VCO, virgin coconut oil.
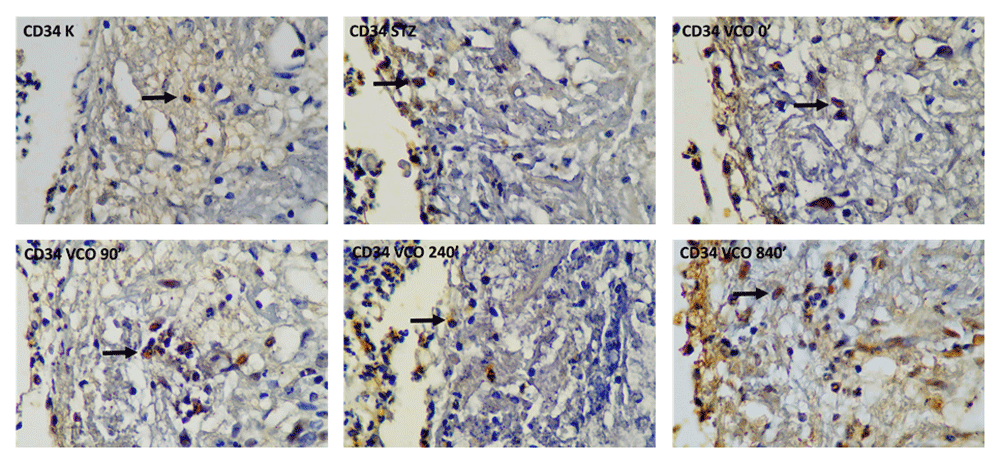
Top row, from left to right: negative control group with negative diabetic status; positive control group with positive diabetic status; non-ozonated VCO therapeutic group. Bottom row, from left to right: 90-minute ozonated VCO therapeutic group; 7-hour ozonated VCO therapeutic group; 14-hour ozonated VCO therapeutic group. The size bar indicates 20 µm.
VCO, virgin coconut oil.
VCO can be produced from fresh coconut or coconut milk, which is rich in medium-chain triglycerides and lauric acid. Lauric acid is a precursor of monolaurin, which can modulate immune cell proliferation. If a wound occurs, the inflammation process commences and immune cell activity increases52,53. Previous research found that VCO caused a significant reduction in ear edema, claw edema and granuloma formation in Sprague Dawley rats54. VCO has been shown to have significant antioxidant effects including increased levels of the superoxide dismutase enzyme in the wound tissue of normal rat17,53. In the case of chronic human skin conditions such as xerosis and atopic dermatitis, VCO shows a significant healing effect55. VCO is also efficacious as a therapy for wound healing and angiogenesis. VCO showed a significant effect on the wound healing of diabetic rat through increased wound closure rate, total protein content, increased collagen synthesis and re-epithelialization. VCO proved to be significantly better than silver sulphadiazine cream in healing diabetic wounds56.
The results of this study show that the application of ozone helps speed up the healing process of a diabetic ulcer. These results are similar to several previous studies. Izadi et al. found that ozone therapy shortened the time needed for diabetic wounds to heal32. A systematic review of several studies reported that ozone therapy reduces the ulcer area and shortens the length of hospitalization compared to control therapies. Ozone therapy was associated with a greater reduction of ulcer area compared to antibiotic therapy26.
Reis et al. reported that ozone provided by a high-frequency device might potentially be useful in the treatment of ulcers, thus contributing to the healing process57. This might be promoted by a decrease in bacterial infection, fibroplasia activation and keratinocyte proliferation. Ozone is a powerful oxidant; if ozone comes into contact with body fluids, it will result in the formation of reactive oxygen molecules and lead to several biochemical events that influence cellular metabolism, tissue repair and microbial infection. Ozone has the potential to promote the activation of transcription factor NF-kB, regulating inflammatory responses, and release of platelet-derived growth factor and transforming growth factor β1 from platelets22,58,59.
The previous study conducted by Kim et al. found an increased intensity of collagen fibers and a greater number of fibroblasts in an acute cutaneous wound for the ozone group60. Furthermore, during a systematic review of the therapeutic use of ozone in wounds, the authors discovered that most of the studies analyzed in their research found stimulation of the healing process (62.2%), followed by improvement in wound appearance (43.5%) and a decrease in pain (17.4%). The mechanism of how ozone improved wound healing might be related to its effect on the growth factors, activation of the antioxidant system, and activation of superoxide dismutase61,62. Besides its effect on the wound healing process, ozone also improves the diabetic condition itself by improving glycemic control, preventing oxidative stress and normalizing organic peroxides, aside from activation of superoxide dismutase as explained above61. This condition might be beneficial to the wound healing process due to the improved condition of diabetes itself. The authors concluded that ozone could be an important treatment option for wounds and may bring numerous benefits to patients61.
In terms of immunohistochemistry parameters, our study found that 14-hours ozonated VCO treatment increased HSP90α, VEGF-A, EGF, bFGF and CD34 levels as measured in our study. Ozone has the potential to increase wound healing by reducing the wound area infection, controlling wound contamination and reducing wound healing time20,63. Ozone has also been found to increase granulation tissue formation, which also increases wound healing speed22. Zhang et al. showed that ozone increases the expression of several wound healing biomarkers, such as VEGF, transforming growth factor-β and platelet-derived growth factor. They also found that ozone improves wound healing in diabetic foot ulcers with a higher wound size reduction in the ozone-treated group25.
As far as we know, there have been no other studies that show any therapeutic modalities, except the direct application of HSP90, that alters the wound healing result64. HSP90α helped to accelerate wound healing by recruiting both epidermal and dermal cells, promoting dermal cell migration, and was able to override the inhibitory effects of hyperglycemia on cell migration in diabetes65. Several studies have found that topically applied HSP90α protein improves wound healing in several kinds of wounds, especially diabetic wounds31,64–66. The main pathology of diabetes is chronic hyperglycemia, which causes destabilization of a key regulator of the HSP90α protein, hypoxia-inducible factor 1 -alpha (HIF-1α)30,64. The HIF-1α pathway is involved in wound healing by helping human dermal fibroblast migration; this is one of the causes of diabetic wound pathology64,66. HSP90α improves wound healing by mediating hypoxia-stimulated human dermal fibroblast motility under normal glycemic conditions and “jumpstarts” cell migration under hyperglycemia conditions31,64. Therefore, HSP90α helps diabetic wound healing by by-passing downregulation of HIF-1α and helps to ‘rescue’ the migration of hyperglycemic cells that otherwise do not respond properly to the environmental hypoxia64. Cheng et al. found that HSP90α can promote wound healing in diabetic rat in a more effective manner65. The middle domain of HSP90α contains the F5 fragment, which acts as a pro-motility factor that affects all three human skin cell types. This HSP90α fragment promotes dermal and epidermal cell migration through LRP-1 or CD91 surface receptors64,65.
Most complications that arise from diabetes are related to vascular alterations caused by diabetes. The pathology of diabetic wound healing is tightly associated with insufficient angiogenesis and reduced VEGF signaling34,35. Seitz et al. found that diabetic rat have decreased VEGF-A levels compared to normal rat67. The low VEGF tissue levels in diabetes patients might be caused by the inability of diabetes patients to appropriately upregulate VEGF expression in response to hypoxia68,69. Galiano et al. found that VEGF-A treated diabetic rat showed accelerated cutaneous wound closure compared to untreated rat. The mechanism of how VEGF facilitates tissue repair is by both increasing vascular permeability, which allows inflammatory cells to enter into the site of injury, and also increasing the migration and proliferation of pre-existing endothelial cells. In the VEGF-A treated diabetic wound model, there is a lot of abundantly vascularized granulating tissue on the wound bed, which causes the wound bed to become very hyperemic. When the VEGF-A treated wounds are almost completely healed, the control wounds were just starting to grow granulating tissue, and the granulation itself did not exhibit hyperemia and vascularization as the VEGF-A treated diabetic wounds did. VEGF treatment also induces neovascularization and cell proliferation in diabetic wounds68.
Diabetes also causes EGF synthesis to be diminished, as shown in diabetic animal models38. EGF is an important factor for wound healing, as it increases the stimulation, proliferation, and migration of several cells related to wound healing, such as keratinocytes, endothelial cells and fibroblasts, facilitates better dermal regeneration, and accelerates the level of wound contraction related to collagen deposition and proliferation of myofibroblasts37,70. Degradation of several growth factors, including EGF, might result in refractory diabetic ulcer wounds. Several studies have shown the benefits of EGF therapy in diabetic wound models. Zhang et al. found that EGF improved diabetic wound healing by: increasing the formation of granulation tissue, collagen uniformity and the extracellular matrix; increasing the number of fibroblasts and improving fibroblast morphology; and also increasing the number of capillaries in the wound. EGF can also induce the migration of inflammatory cells away from the wound, which results in improvement of the wound microenvironment and tissue nutritional status39,39. EGF also has the potential to enhance wound healing and reduce wound healing time, as found by Tsang et al. and Singla et al.40,41
This study found an increase in bFGF levels in wounds treated with ozonated oil compared to nontreated wounds. Re et al. found that ozone increases the basal concentration of bFGF in ozone-incubated platelet-rich plasma71. An early study showed that bFGF improves diabetic wound healing in a diabetic rat model by increasing the rate of cellular infiltration and capillary ingrowth, which might be related to the ability of bFGF to induce angiogenesis in wound healing42,72. bFGF is the primary promoter for cell proliferation73. bFGF contributes to wound healing by inducing angiogenesis, fibroblast proliferation and endothelial cell migration74. A recent study using bioinspired hydrogels with bFGF done by Zhang et al. found that bFGF enhances cell proliferation, re-epithelialization, collagen deposition, and wound contraction in the full-thickness wound healing model. Chronic wound healing is also benefited by the increase of bFGF73.
This study found an increase in CD34 levels in wounds treated with ozonated oil compared to nontreated wounds. CD34 has been used to improve wound healing in diabetic wound models using a CD34 injection direct to the wound itself or systemic injection of CD34 cells. Sivan-Loukianova et al. found that CD34 cells decreased diabetic wound size, accelerated epidermal healing, and rapidly accelerated neovascularization in diabetic wounds, as shown by an increase in blood vessel numbers and diameter75. Cil et al. found that direct application of human umbilical-derived CD34 to the wound margins improves diabetic wound healing by decreasing inflammatory reactions and increasing wound revascularization76. A recent study conducted by Kanji et al. also found that systemically-injected CD34 cells accelerate wound healing in streptozotocin-injected rat. CD34 cells improved re-epithelialization, increased neovascularization, improved granulation tissue formation and increased collagen and myofibroblasts deposition77. Diabetic wounds have decreased angiogenesis and increased matrix metalloproteinases (MMPs), which inhibits proper wound healing in patients with diabetes35. CD34 cells decreased the pro-inflammatory activity of NF-kB, which therefore also reduced the level of MMP-1 expression by inhibiting the recruitment of NF-kB to the MMP-1 promoter site. Therefore, CD34 can reverse the delayed wound healing factors in patients with diabetes. Besides healing effects, the measurement of CD34 cells in the wound margin can predict the healing of diabetic wounds. Thom et al. found that the number of CD34 cells in stained epidermis taken from the wound margin of diabetic wounds from diabetic patients was positively correlated with wound healing itself. Therefore, CD34 measurement in the early weeks of the diabetic wound provides insight into how well the diabetic wound will respond and can be used to predict the healing result of diabetic wounds48.
There are several limitations in this study. First, we only measured macroscopic wound contraction length without analyzing the histological and biochemistry aspects of the wounds themselves. The comparison of wound healing histological events and biochemistry profiles between diabetic control rat and therapeutic group rat might enable further explanation on a mechanistic basis of how ozone improved wound healing in diabetic wounds. Moreover, we only analyzed the wounds until the seventh day and did not measure the events that happened until the wound successfully closed, as the end-result of the wound healing is the restoration of the wound itself. In addition, insufficient literature about bFGF and CD34 in wound healing, especially diabetic wound healing, also leads to difficulty in determining the exact action of bFGF and CD34 in diabetic wound healing and the relation between its increase and its effect in the wound healing process. These could be considered to seek a better understanding of how ozone improved the wound healing process.
As ozonated VCO treatment can increase the levels of several wound healing biomarkers, such as HSP90α, VEGF-A, EGF, bFGF and CD34 levels, its increase might potentially aid the wound healing process. This study is a preliminary study in animals (in this case Wistar rat). Rat can be used as an animal model for several skin diseases, including wound healing. Therefore, we hope that this research can be utilized as a basis for further research on the usage of ozonated VCO in human wounds78.
Ozonated VCO has the potential to increase immunohistochemistry wound healing parameters in a diabetic wound rat model, as shown by the increase of HSP90α, VEGF-A, EGF, bFGF and CD34 levels. The improvement of the wound healing process was proportional to the duration of ozone flow in VCO. A longer treatment period and a longer VCO ozonation duration resulted in higher levels of biomarkers. Further research should be done to improve the application of ozonated oil treatment for diabetic ulcers in everyday practice.
Open Science Framework: Topical ozonated virgin coconut oil improves wound healing and increases HSP90α, VEGF-A, EGF, bFGF and CD34 in diabetic ulcer mouse model of wound healing. https://doi.org/10.17605/OSF.IO/89FQC51.
This project contains the following underlying data:
- Data Tables (raw data in XLSX format)
- Images (original unedited histopathology image files in JPG format)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to express our gratitude to the Department of Dermatology and Venereology, and Center Plasma Research (CPR), Diponegoro University, Semarang.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology of tissue repair, diabetic wounds and inflammatory disorders
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 23 Sep 21 |
read | read | ||
|
Version 2 (revision) 06 May 21 |
read | read | ||
|
Version 1 09 Jun 20 |
read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)