Keywords
Insulin, insulin receptor, glutamate, NMDA receptor, Ca2+, double-negative feedback loop, mitochondria, ATP
Insulin, insulin receptor, glutamate, NMDA receptor, Ca2+, double-negative feedback loop, mitochondria, ATP
Since the discovery of insulin1 and insulin receptors (IR)2 in the brain in 1978, numerous studies have revealed a fundamental role of IR in the central nervous system (CNS). IR-mediated signaling is implicated in the regulation of diverse functions in the CNS, including synaptic plasticity, long-term potentiation and depression, neuroprotection, learning and memory, and energy balance3. Central insulin resistance has been found in neurodegenerative diseases such as Alzheimer’s disease (AD)4,5 and Parkinson’s disease (PD)6, stroke, and traumatic brain injury (TBI)7. Impaired insulin signaling in AD is evident in the activation states of IR and downstream signaling molecules5. Compared with control cases, insulin in AD brains induced 24–58% less activation at the level of IR and 90% less activation of insulin receptor substrate 1 (IRS-1)5. It has been presumed5 that the inhibition of IR activation is mediated by Aβ oligomer-triggered Ca2+ influx, in part by activating N-methyl-D-aspartate receptors (NMDARs)8, followed by a rise in Akt1 pS473 9, which can inhibit insulin-induced IR activation through Thr phosphorylation of the IR β subunit10. Aβ oligomers may activate the NMDAR-gated Ca2+ influx directly11 or indirectly through the intermediate release of glutamate, a ligand of NMDAR11–15. This suggests that the rise in intracellular free Ca2+ concentration ([Ca2+]i), evoked by either Aβ oligomers or glutamate, leads to dysfunctional activation of IR in AD. In the present opinion article, we highlight evidence that IR and [Ca2+]i form a double-negative regulatory feedback loop controlling insulin sensitivity, and mitochondria have a key role in this feedback loop, being involved in adenosine triphosphate (ATP) synthesis and IR activation.
Glutamate serves as the major excitatory neurotransmitter in the CNS. Its excessive accumulation in a synaptic cleft can trigger excitotoxicity, a pathologic process leading to neuronal cell death. Glutamate-induced activation of the NMDAR-gated Ca2+ influx is generally considered central to the development of excitotoxicity16. Prolonged glutamate exposure causes a rapid initial increase in the [Ca2+]i, followed by a larger secondary [Ca2+]i increase concomitant with a decrease in the mitochondrial inner membrane potential (ΔΨm)17–19. We recently found that on Ca2+-induced mitochondrial depolarization, insulin induced 48% less activation of IR (assessed by pY1150/1151) compared with control20. Earlier, we showed that a decrease in ΔΨm can abrogate IR activation18, since the ΔΨm-dependent mitochondrial signal at complex II is involved in the activation of IR in neurons21–23. Thus, the glutamate-evoked increase in [Ca2+]i, followed by the drop in ΔΨm, leads to the inhibition of insulin-induced activation of IR.
Normally, the NMDAR-gated Ca2+ influx is counterbalanced with Ca2+ efflux, which is governed by plasma membrane Ca2+ ATPase and the Na+/Ca2+ exchanger (NCX)24,25. NCX-mediated Ca2+ efflux is also ATP-dependent, since NCX exchanges one Ca2+ for three Na+, and the three Na+ are then pumped out by the Na+/K+ ATPase at the expense of one ATP. In excitotoxicity, prolonged stimulation with glutamate leads to ATP depletion and an abnormal rise in [Ca2+]i, since the massive Ca2+ influx is no longer counterbalanced by Ca2+ efflux26. Therefore, maintenance of ATP production is crucial for preventing the rise in [Ca2+]i in excitotoxicity. We found recently that pre-treatment with insulin prevents neurons from glutamate-evoked ATP depletion due to its protective effect on spare respiratory capacity (SRC), a measure that relates to the amount of extra ATP that can be produced via oxidative phosphorylation in case of increased energy demand19. The effect of insulin on SRC relates to its action on mitochondrial metabolism. It has long been known that the tricarboxylic acid cycle is the intracellular site of insulin action and that insulin acutely stimulates succinate oxidation at mitochondrial complex II26,27. Succinate oxidation at mitochondrial complex II has been identified recently as the main source of SRC28. In line with this, insulin prevented the glutamate-evoked rise in [Ca2+]i in our experiments with glutamate excitotoxicity19.
Collectively, this evidence suggests that a double-negative regulatory feedback loop exists between IR and [Ca2+]i. The glutamate-evoked rise in [Ca2+]i inhibits activation of IR and, vice versa, insulin-induced activation of IR inhibits the glutamate-evoked rise in [Ca2+]i (Figure 1a).
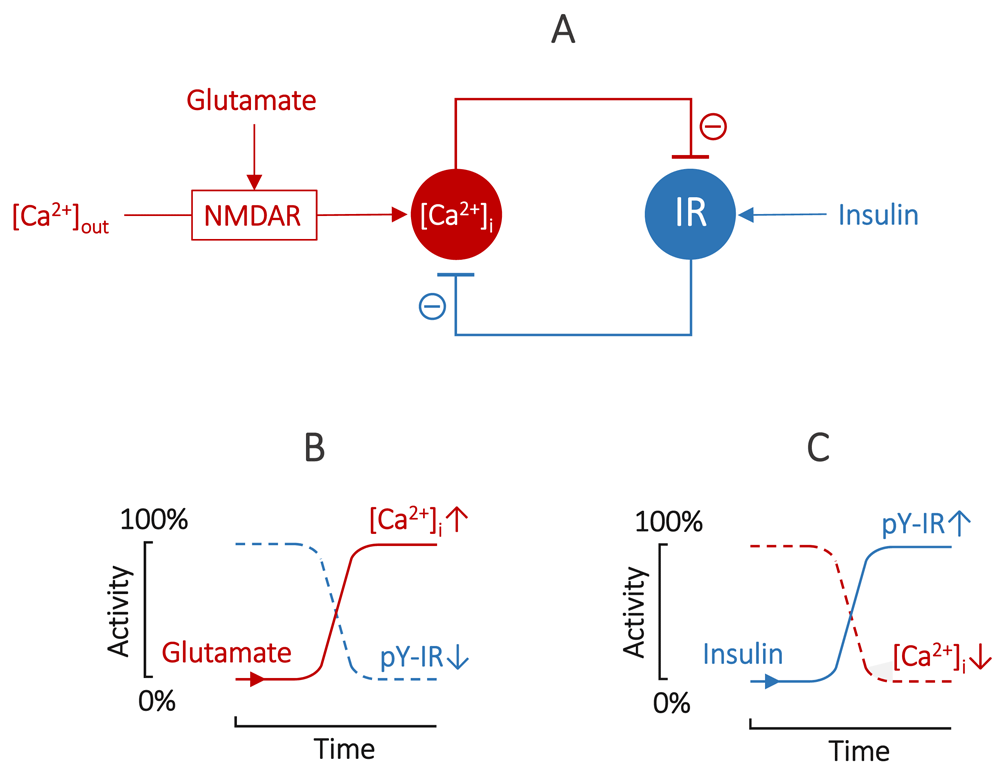
(A) glutamate triggers NMDA receptor–gated Ca2+ influx, inhibiting IR activation, and insulin triggers activation of IR, inhibiting [Ca2+]i rise; (B) glutamate-triggered stable steady state with high [Ca2+]i and nonactive IR (pY-IR↓); (C) insulin-triggered stable steady state with active IR (pY-IR↑) and low [Ca2+]i.
In theory, a double-negative feedback loop generates bistability29. Thus, a stable steady state could exist with high [Ca2+]i and nonactive IR (Figure 1b), or with active IR and low [Ca2+]i (Figure 1c), but no stable steady state is possible with both high [Ca2+]i and active IR. Such a circuit could toggle between a high [Ca2+]i state and an active IR state in response to glutamate and insulin, respectively.
This double-negative feedback loop model predicts that any condition leading to an increase in [Ca2+]i may trigger insulin resistance. It appears to explain why central insulin resistance is implicated in the pathogenesis of disorders such as AD4,5, PD6, stroke, and TBI7, with which glutamate excitotoxicity is a comorbid condition30. The model also predicts that any intervention aiming to prevent Ca2+ influx of or enhance efflux of Ca2+ from neurons, thereby maintaining low [Ca2+]i, may be useful for treating central insulin resistance. Given that Ca2+ efflux is ATP-dependent, any intervention directed to enhance ATP production in neurons may be especially useful to improve insulin sensitivity in the brain.
No data are associated with this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Systems biology, Liver metabolism, modeling signaling pathways
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: cerebral vascular disease
Is the topic of the opinion article discussed accurately in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
No
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Partly
References
1. Zhao WQ, De Felice FG, Fernandez S, Chen H, et al.: Amyloid beta oligomers induce impairment of neuronal insulin receptors.FASEB J. 2008; 22 (1): 246-60 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: glutamate signaling, Alzheimer's disease, Parkinson's disease, insulin signaling, gerontology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 13 Jan 21 |
read | read | |
|
Version 1 12 Jun 20 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)