Keywords
urinary tract infections, prostate-specific antigen, men, prostatitis, antibiotic treatment duration
urinary tract infections, prostate-specific antigen, men, prostatitis, antibiotic treatment duration
In the new version we have modified the first sentence in the Discussion section to make it more factually accurate.
See the authors' detailed response to the review by Veeravan Lekskulchai
See the authors' detailed response to the review by Aneesh Basheer
Urinary tract infections (UTIs) in men are generally considered to be complicated UTIs because of increased prevalence of underlying structural and functional abnormalities1. One such abnormality is the possibility of involvement of the prostate gland during an episode of UTI. Symptomatic involvement of the prostate gland by acute bacterial infection, known as acute bacterial prostatitis (ABP), classically presents with fever and systemic symptoms along with pelvic pain and infravesical obstruction2. However, even in the absence of prominent voiding and storage symptoms, subclinical involvement of the gland is possible in men with febrile UTI (fUTI). A study of Swedish men with fUTI provided the major evidence for this, in the form of increased serum prostate-specific antigen (PSA) levels and prostatic volume during an episode of UTI3. Another study using 111indium-labelled leukocyte scintigraphy found that, although often clinically unrecognized, the prostate was involved in most cases of fUTI and acute pyelonephritis in men4.
Differentiating fUTI with subclinical prostatic involvement from classical ABP is important. First, some experts recommend that antibiotic treatment in men with fUTI should not only sterilize the urine but also achieve sufficient concentrations in the prostate. Fluoroquinolones and co-trimoxazole were recommended as optimal choices to achieve this aim5,6. If the increased levels of PSA are truly indicative of ABP, implementing this guidance would be pragmatically challenging in settings where antimicrobial resistance, especially to fluoroquinolones, is very common among uropathogens and other appropriate oral options are not available7. Second, while it is generally agreed that ABP requires antibiotic treatment for at least two to four weeks to prevent chronic prostatitis8, it is unclear whether subclinical prostatic involvement would also necessitate a longer treatment. We, therefore, conducted the present study to answer the following research questions — i) What is the frequency of prostatic involvement in men with fUTI, and ii) Is prostatic involvement associated with recurrence of UTI?
The study protocol was reviewed and approved by the Institute Ethics Committee (Human Studies) of Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry (JIP/IEC/2016/29/970). Written informed consent was obtained from all study participants.
We conducted a prospective observational study in the medical wards of Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), a tertiary care hospital in Southern India, during July 2016 and May 2018. During this study period, we screened all consecutive male patients aged 18 years or over admitted under the Department of Medicine as suspected cases of fUTI for eligibility to participate in the study. We defined fUTI as documented fever of at least 38°C with at least one symptom or sign referable to the urinary tract such as dysuria, frequency, urgency, flank pain or renal angle tenderness. All patients had evidence of microscopic pyuria (>5 pus cells/hpf) or urine dipstick positive for leukocyte esterase. We excluded patients with catheter-associated UTI, urological procedures or surgery in the past four weeks, diagnosed with prostate carcinoma, or significant upper urinary tract obstruction evidenced by gross hydroureteronephrosis (HUN).
After obtaining written informed consent, the investigator (TSA) performed a standardized clinical evaluation. A digital rectal examination (DRE) was done to look for prostatic enlargement, tenderness or bogginess. Blood samples were drawn within 48 hours of admission for serum PSA and high sensitivity C-reactive protein (hs-CRP) estimation, and serum was stored at -80°C for batched analysis. Since DRE may lead to an increase in serum PSA levels, blood samples were drawn before performing DRE. Serum PSA levels were estimated in duplicate using a two-site immune-enzymatic sandwich assay that uses a mouse monoclonal anti-PSA antibody (Cat. No. 37200, Hybritech Prostate Specific Antigen, Beckman Coulter, Fullerton, CA) as described for the National Health and Nutrition Examination Survey 2001–20029. Serum hs-CRP was estimated in duplicate using a solid phase direct sandwich assay which uses a monoclonal antibody (Cat. No. CR120C, Calbiotech. Inc., El Cajon, CA) as per the manufacturer’s instructions. All patients underwent ultrasonographic examination of kidney, ureter and bladder. As soon as the patients became clinically stable, a transrectal ultrasound (TRUS) examination of the prostate and seminal vesicles was performed using Famio 8 SSA-530A (TOSHIBA), PVQ 641V 6 MHz probe by a urology senior resident. We collected data on the antibiotic regimen started, duration of therapy and clinical course during hospital stay.
At the first follow-up assessment after one month of treatment completion, serum PSA estimation was done in all patients. In those with persistent fever or urinary tract symptoms, urine dipstick leukocyte esterase test and microscopic examination were done. In those with evidence of significant pyuria, urine culture was repeated. At the second follow-up visit after three months of treatment completion, clinical evaluation and repeat measurements of serum levels of PSA and hs-CRP were done. TRUS was also repeated to re-assess the size of prostate and to assess resolution or persistence of inflammation. A baseline serum PSA level above 97.5th percentile of decade-specific serum PSA levels in healthy Indian men was considered to be elevated. We calculated this upper limit cut-off based on a previous study of serum PSA levels among 1300 healthy adult Indian men10. We used the decade-specific mean and standard deviation (SD) of PSA values and calculated the 97.5th percentile as mean + 1.96 SD. We defined prostatic involvement as per the criteria suggested by Ulleryd et al.3. A reduction of serum PSA by >25% irrespective of the initial PSA level, and/or a decrease in prostatic volume by >10% between the acute phase and the follow-up after 3 months was taken as evidence of prostatic involvement.
Assuming that 80% of patients would have evidence of prostatic involvement3, 64 patients were required to estimate this proportion with 10% absolute precision.
We summarized categorical variables as n (%) and continuous variables as mean±SD or median (IQR) as appropriate. We applied the Wilcoxon signed rank test to assess the change in serum PSA and serum hs-CRP levels at three months compared to baseline. We performed the Friedman test to analyze the trend of serum PSA at admission, one month and three months. We applied the Wilcoxon rank sum test to compare baseline serum PSA and change in serum PSA levels at three months between patients with and without clinical features suggestive of prostatic involvement. We tested the correlation between baseline serum PSA levels and fall in its levels by three months and that between baseline serum PSA levels and the change in serum hs-CRP levels by three months using Spearman’s rank correlation. All analyses were performed using the statistical software package Stata/IC 12.1 for Windows, StataCorp LP, College Station, Texas, USA. All tests were two-sided, and P <0.05 was considered statistically significant. We used GraphPad Prism version 8.3.0 for Windows, GraphPad Software, San Diego, California USA for graphical summaries.
Between July 2016 and May 2018, we screened 91 men with a diagnosis of fUTI and included 64 patients; 50 patients completed follow-up assessments at one month and three months. Figure 1 depicts the flow of subjects through the study. Clinical and laboratory characteristics of the study subjects at baseline are summarized in Table 1. Notably, 16(25%) of 64 patients had presented with obstructive urinary symptoms and 25 (39%) patients had prostatic tenderness on DRE.
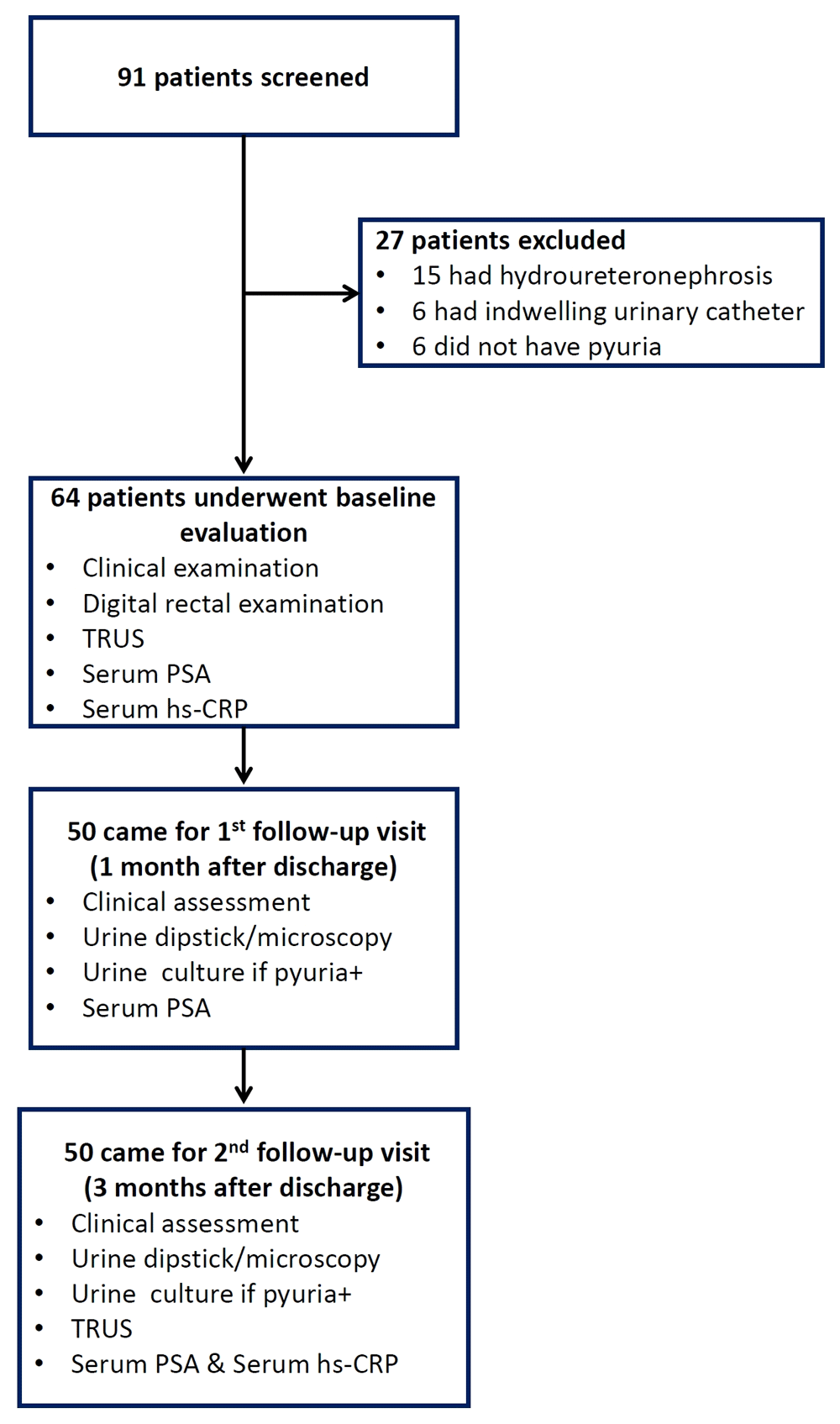
TRUS, transrectal ultrasound; PSA, prostate-specific antigen; hs-CRP, high sensitivity C-reactive protein.
Mean duration of fever was 6.7±4.4 days; 15 (23%) of 64 patients had received antibiotics prior to study enrollment. Using a strict diagnostic criteria of fever, dysuria, urinary retention and prostatic tenderness on DRE, eight (12%) of 64 patients could be classified as cases of ABP. Urine culture was sent prior to the first dose of antibiotic after admission in 45 (70%) of 64 patients. Eschericia coli was the major uropathogen, isolated in 25 (86%) of these 45 patients.
Empirical antibiotic regimens used in the study population (N=64) were ceftriaxone in 28 (44%), amikacin in 21 (33%), a combination of ceftriaxone and amikacin in seven (11%), cefaperazone/sulbactum in three (5%), a combination of piperacillin/tazobactum and amikacin in three (5%) patients and meropenem in one (2%) patient. A patient with Candida tropicalis fungemia and prostatic abscess was treated with fluconazole for 28 days. Modification of the empirical regimen based on susceptibility was done in six (9%) patients. Mean duration of antibiotic therapy was 8.6±3.6 days.
Of the 64 patients included, 50 patients came for first follow-up visit after one month of treatment completion. Among them, persistent lower urinary tract symptoms (LUTS) were present in seven (14%) patients. One of them (patient 1), who had fungal prostatic abscess at initial admission, gave a history of recurrent fever. He had significant microscopic pyuria and urine culture showed significant growth of E. coli, which was susceptible to amikacin. He was re-admitted and was treated with amikacin for seven days. Of the remainder, two patients had significant microscopic pyuria, while four did not. Urine cultures in the two patients with pyuria showed Klebsiella spp in one patient (patient 2; treated with nitrofurantoin on ambulatory basis). The third patient’s (patient 3) urine culture was contaminated, and a repeat culture was sterile. Since his LUTS improved significantly with increased fluid intake, he was not treated with antibiotics.
The same set of 50 patients attended the three months follow-up. Of note, the three patients (patient1, 2 and 3) who had LUTS and microscopic pyuria during first follow-up visit continued to be symptomatic at this visit too, although none was febrile. They continued to have significant pyuria at this visit. While the repeat urine culture of patient 1 was sterile, patients 2 and 3 had significant bacteriuria, the organisms were different from previous cultures (Enterobacter spp and Enterococcus spp, respectively). No antibiotic therapy was prescribed in these three patients at this juncture. They were advised to maintain good hydration. All three patients had significant resolution of symptoms subsequently. Details of these patients are presented in Table 2.
At admission, 24 (38%) of 64 patients had elevated serum PSA values. Among the 50 patients who followed up, a significant decrease in serum PSA levels compared to the baseline was noticed at 3 months (Table 3). The fall in serum PSA levels at 3 months was strongly correlated to the baseline serum PSA value (Spearman’s rho 0.93; P <0.001; Figure 2, panel B.) Of the 50 patients, 47 (94%) had prostatic involvement as per the pre-defined criteria based on significant change in serum PSA levels. This also included 29 patients whose baseline serum PSA was not elevated.
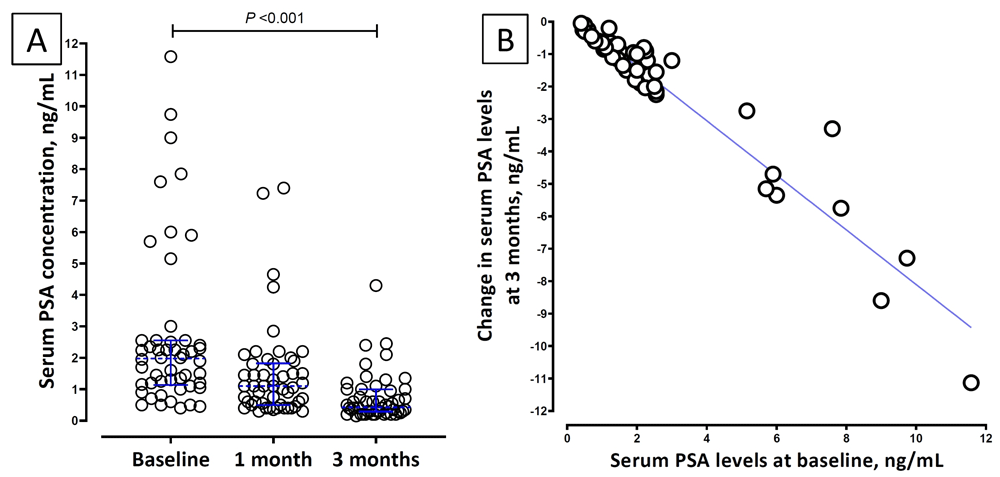
Panel A: Dotplot of serum prostate-specific antigen (PSA) levels at baseline, one month and three months of follow-up. Dotted blue lines across the data points depict the median and the error bars depict interquartile range. Panel B: Correlation between baseline serum PSA level and the change in PSA levels at three months.
Serum hs-CRP levels also decreased significantly at three months compared to baseline (Table 3, Figure 3). There was no correlation between serum PSA levels and hs-CRP levels either at baseline or at three months. The change in serum PSA levels over 3 months had no correlation with the change in serum hs-CRP levels (Spearman’s rho 0.19; P=0.188).
TRUS was done in 64 patients at baseline, within two (2–4) days of hospitalization. There were no procedure-related complications. The most significant finding was the presence of prostatic abscess in one patient. Other findings on TRUS are presented in Table 3. Follow-up TRUS examination was done in 50 patients, 95±7 days after hospital discharge. Of the six patients who had focal hypoechogenicity at admission, three continued to have the finding at three months, while new hypoechogenic lesions were noticed in two other patients. However, none of the patients who had these lesions were symptomatic at three months.
A decrease of in prostatic volume ≥10% was observed in 24 (48%) patients. The change in serum PSA levels at three months did not correlate with change in prostatic volume.
Serum PSA levels at baseline did not differ significantly between patients with/without clinical features suggestive of prostatic involvement such as urinary retention, lower abdominal pain, prostatic tenderness on DRE or a possible diagnosis of ABP. Similarly, none of these clinical features were associated with the change in serum PSA levels compared to the baseline (Table 4).
| Clinical finding | Baseline serum PSA level, ng/mL | P value | Change in serum PSA level at three months, ng/mL | P value | ||
|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | |||
| Urinary retention | 2.07 (1.45 to 4.75) | 2.15 (1.03 to 2.9) | 0.40 | -1.35 (-2.04 to -0.9) | -1 (-0.2 to -0.65) | 0.51 |
| Lower abdominal pain | 2.0 (1.05 to 3.05) | 2.4 (1.45 to 3) | 0.29 | -0.95 (-2.0 to -0.65) | -1.2 (-2.25 to -1.1) | 0.91 |
| Prostatic tenderness | 2.1 (1.05 to2.7) | 2.2 (1.2 to 4.9) | 0.63 | -1.35 (-2.07 to -0.6) | -1.05 (-2 to -0.7) | 0.96 |
| Possible ABPa | 2.2 (1.57 to 4.2) | 2.15 (1.13 to 3.02) | 0.61 | -1.5 (-4.7 to -0.95) | -1.05 (-0.2 to - 0.67) | 0.39 |
We found that about a third of men with fUTI requiring hospitalization had elevated serum PSA levels at presentation, and most men with fUTI requiring hospitalization had at least 25% decrease in serum PSA level at 3 months. Further, nearly half of them had a decrease in prostatic volume on follow up. However, only a handful of them had clinical findings suggestive of prostatic involvement, and recurrence following treatment was uncommon.
Our findings are in agreement with the seminal study by Ulleryd et al.3. Although there are a few more studies on PSA levels in men with fUTI, these studies had enrolled patients with ABP11,12. The median (IQR) serum PSA levels at admission in our patients was 2.15 (1.18–3.02) ng/mL, which is lower compared to the study by Ulleryd et al., which was 14 (range 0.54–140) ng/mL. We used a chemiluminescent immunoassay method, while Ulleryd et al. used a monoclonal fluoroimmunoassay. While mild assay-related variations are possible, the main reason for lower PSA levels in our study could be because of ethnic variations in PSA levels. Studies from India show that the mean serum PSA values in Indian men are lower compared to the Western population13–15.
Conventionally, elevated PSA levels and a change in prostatic volume have been proposed as definitive evidence of prostatic involvement in men with fUTI3,6. Based on this premise, often it is contended that fUTI in men should be treated with antibiotics for a duration of at least two weeks. However, interpreting changes in PSA levels and prostatic volume as reliable evidence of ‘prostatitis’ is questionable. ABP is a clinically defined entity classically characterized by fever, systemic symptoms, pelvic pain and urinary tract symptoms such as dysuria, urinary frequency, and urinary retention16. Although it is known that PSA levels become elevated in men with ABP, the converse may not be true. Our findings indicate that it might be fallacious to equate pauci-symptomatic elevation of serum PSA levels as definitive evidence of prostatitis due to following reasons.
First, only a minority of patients with such changes actually had clinical findings to suggest prostatic involvement, and the PSA levels and prostatic volume changes were not related to prostatic symptoms. Second, we did not find a correlation between hs-CRP levels and the elevation in PSA levels and the change in prostatic volume. Notably, Ulleryd et al. also did not find a correlation between elevated serum PSA and markers of systemic inflammation. Third, despite the fact that 80% of patients were treated with antibiotics for seven days duration, recurrence of UTI was uncommon. Thus, while we confirm the findings that transient elevations in PSA levels and prostatic volume are very common in men with fUTI, we disagree with the interpretation of the clinical significance of these subclinical changes. It is quite possible that the elevated PSA levels indicate a physiological response to bacterial infection rather than indicating a pathological disease process. Townes et al. found that epithelial expression and release of PSA was increased by E. coli challenge17. A few other studies also show that elevated PSA levels represent an enhanced prostate innate host defence18,19. Serum PSA levels also rise during episodes of sexually transmitted infections and may remain elevated for several months after effective antibiotic therapy20. Other non-genitourinary infections like infectious mononucleosis and chikungunya also cause elevated serum PSA levels21,22. It is possible that elevated PSA level is a non-specific response to systemic inflammation caused by prostate cell damage and increased vascular permeability.
We found the presence of focal hypoechoic areas in prostate in a small proportion of patients, the significance of which is unclear — these patients also had good response to treatment and none had LUTS on follow up. Horcadaja et al. observed hypoechoic lesions in about 25% of patients with ABP23; these lesions persisted in about one-third of patients after antibiotic therapy for one month.
The practical application of clinically distinguishing fUTI from ABP is mainly to decide on the choice and duration of antibiotics. It would be fallacious to justify the need for prolonging the antibiotic treatment based solely on biochemical and TRUS changes which might possibly suggest prostatic involvement. While clinicians generally agree on the need to treat patients with ABP for at least two weeks, it needs to be pointed out that this duration is not based on good quality evidence24. In addition, considerable heterogeneity exists in the diagnosis and management of ABP among various clinical departments25. Even though a shorter duration of antibiotics was associated with an increased risk of recurrent prostatitis in observational studies, it could be because those patients had clinically manifest ABP and not just biochemical and/or ultrasonographic changes26. Indeed, even in ABP, some experts believe that the role of shorter treatment duration needs to be explored27. This is a very important aspect since longer treatment durations have been paradoxically associated with increased late recurrences of UTI in the outpatient setting28. In addition, longer antibiotic treatment durations do not augur well with the principles of antibiotic stewardship29.
Very few clinical trials have addressed the issue of optimal treatment duration for fUTI in men without features of ABP. A recent trial from the Netherlands found that shorter duration resulted in lower clinical cure rates at short term in men30. Prostatic involvement was attributed as a possible reason for this. However, clinical cure at 70–84 days did not differ between genders, and shorter treatment did not result in more recurrence in men on long term. Another smaller trial from India comparing non-fluoroquinolone antibiotic therapy for seven or 14 days found no difference in re-treatment rates between males and females31. Average antibiotic treatment duration in the present study was less than 10 days. Yet, we did not find significant short-term recurrence of fUTI in them.
Possible limitations of our study are: i) it would have been more informative if we had longer follow-up and assessment for chronic prostatitis in the study population; ii) measurement of prostatic volumes potentially could have been affected by inter-observer variability; and iii) we did not collect data on glandular vascularity, which could indicate the presence of inflammation32.
In conclusion, increase in serum PSA levels and certain ultrasonographic findings, which might possibly indicate subclinical prostatic involvement, were common among men with fUTI. However, the clinical significance of these changes is uncertain.
Figshare: Prostatic involvement in male UTI. https://doi.org/10.6084/m9.figshare.12286865.v233
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors thank all the participating patients and the staff at JIPMER for their kind co-operation. The authors thank Dr. Tamilarasu Kadhiravan, Additional Professor, Department of Medicine for critical appraisal of the study findings and help with the figures in the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases, tropical medicine, hematology, evidence based medicine, medical education
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases, tropical medicine, hematology, evidence based medicine, medical education
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Pathology, Clinical Chemistry, Clinical Toxicology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases, tropical medicine, hematology, evidence based medicine, medical education
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Pathology, Clinical Chemistry, Clinical Toxicology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 10 Nov 20 |
read | |
|
Version 2 (revision) 27 Oct 20 |
read | read |
|
Version 1 16 Jun 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)