Keywords
Transforaminal, caudal, epidural steroid injections, lumbosacral radicular pain, randomized controlled trial.
This article is included in the All trials matter collection.
Transforaminal, caudal, epidural steroid injections, lumbosacral radicular pain, randomized controlled trial.
The 1 st version was revised because there demonstrated one categorical outcome being > 50% pain relief from previous study but Bicket MC et al. (Pain Pract 2017;17(8):1058-65 ) showed the impact guidelines state that 30% or more pain relief is clinically meaningful as Dr. Cohen SP’s recommendation. Therefore, we considered showing the proportion of people who also obtained > 30% pain relief in version 2 and found more effective and showed significant pain relief in combined group(TC group) at 3 months follow ups but insignificant in subgroup allocation. Moreover, 2nd version showed two fluoroscopic imaging that showing the flow of contrast which determine of deposition of steroid as Dr. Mohamed AH’s suggestion. Furthermore, more detail in figure 3 showed that two participants who were excluded due to wrong allocation from progressive weakness and scheduled for surgery and alternated figure legends in figure 2 and 3 (version 1) were edited and showed in Figure 4 and 5 (version 2) as peer review’s guidance for good quality of the research.
See the authors' detailed response to the review by Jatuporn Eiamcharoenwit
See the authors' detailed response to the review by Steven P. Cohen
See the authors' detailed response to the review by Koravee Pasutharnchat
See the authors' detailed response to the review by Abdul Hadi Mohamed
Chronic lumbosacral radicular pain (CLRP) is a common condition in pain and spine centers. Treatment is challenging among patients who do not respond to either medication or physiotherapy and epidural steroid injection (ESI) is one commonly used intervention to alleviate radicular symptoms1. These inhibit the synthesis of prostaglandins, interrupting nociceptive c fibers and reducing edema surrounding the nerve root2–4.
Different approaches of ESI are available, namely, transforaminal ESI (TFESI), interlaminar ESI (ILESI), and caudal ESI (CESI)5–9. The effectiveness of these three injected approaches has been shown. Related studies10–12 have reported that TFESI was more beneficial than CESI regarding pain relief in herniated disc or radicular pain13. However, one recent systematic review and meta-analysis revealed TFESI could be weakly recommended over CESI14. Furthermore, one retrospective study showed adjunctive CESI on TFESI significant relieved more pain than only TFESI15. Unfortunately, a prospective study has not been conducted of the additional effects of combining the epidural steroid approach. Consequently, this study aimed to compare the effectiveness of pain relief and functional outcome between additional CESI to TFESI and TFESI separately in chronic lumbosacral radicular pain in a prospective randomized study and also to investigate possible complications during injection.
The study comprised a prospective, single center, randomized, double blind, active-controlled parallel group. Permission to conduct this study was granted by the Institutional Review Board of the Royal Thai Army Medical Ethics Committee and registered with the Thai Clinical Trials Registry on 11 November 2017 (TCTR20171101002).
This study was conducted from November 2017 to January 2019. In total, 54 patients who met inclusion criteria were recruited. Patients attending the PMK Pain Treatment Center, Phramongkutklao Hospital were informed by a nurse anesthetist about the study. Patients who indicated an interest then provided written informed consent. The inclusion criteria comprised patients aged 18 to 80 years old with a history of chronic lumbosacral radicular pain (longer than six months) having a diagnosis of either symptomatology or physical examination correlated using magnetic resonance imaging (MRI) and unsatisfactory pain control with either medication or physiotherapy. The exclusion criteria comprised patients presenting significant neurological deficit or cauda equina syndrome, no absolute contraindications to intervention from MRI such as discitis or spinal infection, coagulopathy, psychiatric problem, pregnancy, language barrier or history of allergy to local anesthetics, triamcinolone and contrast media.
All patients were interviewed and physically examined by only one pain physician. Pain characteristics were documented and randomly allocated in two groups equally using a randomization with block size of four from a computer-generated table and concealed envelope. The random numbers were kept sealed and opened by a nurse anesthetist uninvolved in this study. Those in the TC group received CESI in addition to TFESI, and the T group received only TFESI. All participants and one nurse anesthetist, trained as outcome assessor were blinded to the study group.
The intervention was performed as a day surgery using local anesthesia. The treatment level determined for supraneural transforaminal approach was based on clinical symptoms correlating with MRI. All patients were placed in the prone position, then pulse oximetry and noninvasive blood pressure were monitored. The lower back and buttocks areas were cleaned using sterile fashion technique.
A C-arm fluoroscope (9900 Elite, Super C, OEC, UT, USA) was adjusted and rotated obliquely 20 to 25 o ipsilateral to the affected side and 0 to 10 o cephalo-caudad tilt until aligned with the superior vertebral end plate. The needle entry points were identified and the skin was infiltrated with 4 to 6 mL 1% lidocaine. A Quinke needle (22-G, 10 cm long) (Unilever, Japan) was inserted in the direction of the radiation beam. The supraneural transforaminal technique was performed, and the tip of the needle was placed below the pedicle and within the upper half of the intervertebral foramen in the lateral image. Then 0.5 to 1 ml of nonionic contrast media (Omipaque 300, GE Healthcare, Shanghai, China) was injected via extension tubing to confirm the needle’s location at the target area under real time fluoroscopy. The caudal epidural space was identified using fluoroscopic guidance in the lateral position and then a 16-gauge introducer Touhy needle (Epimed International RK, Epimed International, Johnstown, NY, USA) was inserted through the sacral hiatus into the caudal space and an epidural catheter was inserted using Touhy needle. Then 0.5 to 1 ml of nonionic contrast media was injected via epidural catheter (Epimed International RK, Epimed International, Johnstown, NY, USA) under real time imaging to confirm the desirable vertebral level and covered target site on both groups as shown in Figure 1 and Figure 2. T-group underwent 0.08% Levobupivacaine (Abbvie S.r.l., Italy) plus 40 mg of triamcinolone (L.B.S. Laboratory Ltd., Bangkok, Thailand) in a total volume of 3 ml via only the intervertebral foramen. Those in the TC group underwent 3 ml of 0.08% Levobupivacaine plus 40 mg of triamcinolone using the transforaminal approach combined with 10 ml of 0.025% Levobupivacaine plus 40 mg of triamcinolone via caudal epidural catheter.

The primary outcome was an effective response to treatment, predefined by at least a 30% reduced verbal numerical rating scale (VNRS; 0-100) from baseline16 between group comparisons. The secondary result was functional outcome, measured by improved Oswestry Disability Index (ODI, Thai version 1)17 at least 15 points from baseline. All participants were completely supervised and evaluated by blinded outcome assessor before the procedure, and then subsequently at 1, 3 and 6 months after procedure when attending the outpatient department of PMK Pain Treatment Center.
The sample size was calculated based on the related study of Ploumis A et al.12. The probability of significantly reduced pain from TFESI was 0.90, whereas the probability of significantly reduced pain from CESI was 0.545. The result indicated 24 patients were required for each group to reach a significance level of 0.05, the power of study was set at 80%, and we added 10% more for loss to follow-up. The final number of participants totaled 27 per group. All analyses and summaries were performed with Stata, Version 13/SE (StataCorp, 2013, College Station, TX, USA). A P value <0.05 was considered statistically significant. Descriptive statistics for continuous variables was presented as mean and standard deviation (SD) for sufficiently normally distributed variables. For nominal data, absolute and relative frequencies were displayed for each category. Independent t-test and Chi-square test were performed to compare the differences between groups in continuous variables and categorical variables, respectively. Multilevel mixed linear regression was performed to compare the Verbal Numerical Rating Scale (VNRS) and ODI Questionnaire change over the study period between both groups. A random intercept for the patients was included in the model to account for the cluster structure of the data (two-level models).
In total, 54 eligible patients were enrolled and allocated in equal groups of 27. Two participants were more excluded due to wrong allocation from progressive weakness and scheduled for surgery in the T group. Consequently, 25 patients remained in the T group and 27 in the TC group as shown in Figure 3. No differences were found regarding demographic data, sex, weight, clinical diagnosis, level of pain dermatome, preprocedural VNRS and ODI before intervention as presented in Table 118. Mean baseline VNRS was high in both groups; 74.8 ± 16.8 and 69.6 ± 15.1 in the T and TC groups, respectively.
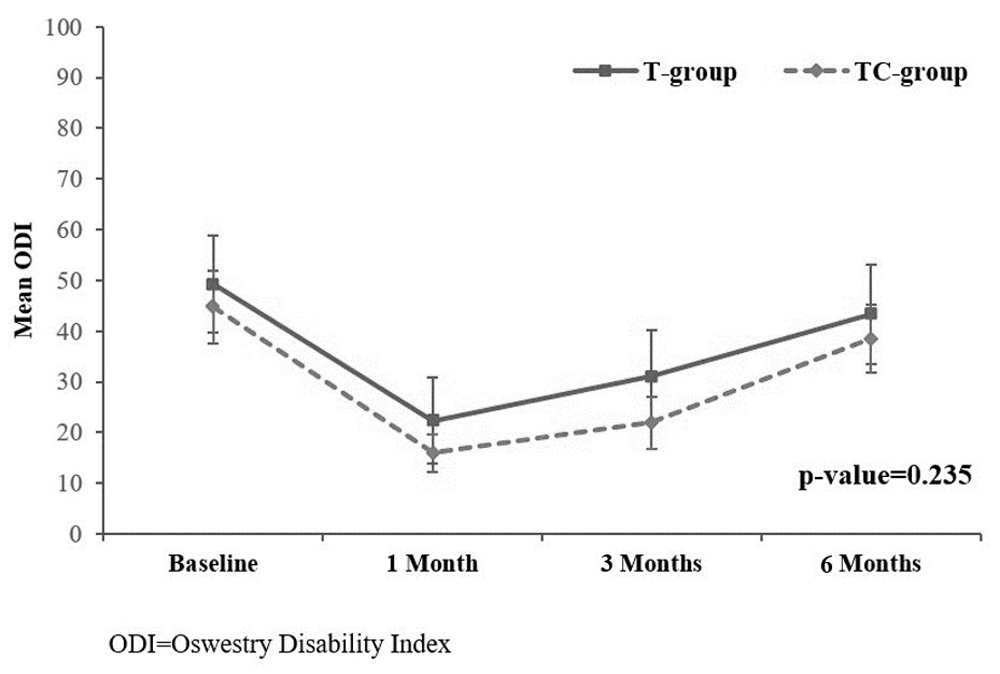
Overall, average VNRS was significantly reduced from baseline after receiving the procedure at 1, 3 and 6 months in both groups (P-value <0.05). However, the study showed significant differences between group comparisons at 1 and 3 months (P-value=0.009 and 0.044), respectively) as shown in Figure 418. Moreover, average ODI was significantly improved from baseline at 1, 3 and 6 months in both groups. Nonetheless, no significant difference was found in average ODI over the study period between group comparisons (p=0.235) as presented in Figure 518.

The number of patients, responding to treatment, was measured by decrease in VNRS of 30% or greater from baseline at each follow-up period, as presented in Table 218. This study showed the TC group exhibited more patient responses to the procedure and a significant difference than T group at 3 months follow up (p=0.01). However, there was no significant difference between group comparison in subgroup allocation (p>0.05), as shown in Table 318 . Interestingly, the TC group were more satisfied with the treatment outcome in spondylolisthesis and failed back surgery syndrome at 3 months, although not statistical significance (p=0.07 and p=0.08, respectively).
| TFESI (n=25) | TFESI+Caudal (n=27) | p-value | |
|---|---|---|---|
| 1 month | 20 (80.0%) | 25 (92.6%) | 0.184 |
| 3 months | 13 (52.0%) | 23 (85.2%) | 0.010 |
| 6 months | 7 (28.0%) | 7 (25.9%) | 0.866 |
Functional outcome assessed by the number of patients with improved ODI at least 15 points at each follow-up period showed no significant difference between groups, classified by either radicular symptoms or etiology as shown in Table 4 and Table 518.
| TFESI (n=25) | TFESI+Caudal (n=27) | p-value | |
|---|---|---|---|
| 1 month | 16 (64.0%) | 21 (77.8%) | 0.273 |
| 3 months | 12 (48.0%) | 18 (66.7%) | 0.173 |
| 6 months | 7 (28.0%) | 10 (37.0%) | 0.488 |
Additionally, no serious complications, such as neurological deficit, was reported during the course of the study.
This constituted the first prospective study to compare clinical outcomes of combined CESI to TFESI (TC group) and TFESI alone (T group) to alleviate chronic lumbar radicular pain.
This study showed combined CESI with TFESI provided more effective pain relief than TFESI separately at 3 months. Moreover, a trend was shown for higher pain relief in the TC group among spondylolisthesis patients ( total of patients in the TC group compared with 3 of 7 patients in the T group) and fail back surgery syndrome (5 of 6 patients in the TC group compared with 1 of 5 the patients in the T group) at 3 months in subgroup allocation, without significant difference in which prior studies reported the mechanism of radicular pain in spondylolisthesis was usually from mechanical compression resulting in inflammatory changes in the enclosing nerve root and venous and arterial flow disability19,20. Accessing the epidural space of the supraneural TFESI is relatively difficult in a severely degenerated and narrowed foramen13,21. Furthermore, the injected volume of lumbar TFESI is likely to influence the results. Prior studies have reported that larger injected epidural volumes provide effective pain relief16,22 and a larger injected volume can lavage waste products from the epidural space, reducing the abnormal signal of the offending nerve and increasing blood flow to the ischemic nerve23. Desai et al.24 confirmed that the more vertebrae covered by the injected volume the better the outcome, and Furman et al.25 commented that a larger injected volume was needed for failed back surgery patients. Unsurprisingly, the TC group showed trending toward more pain relief in spondylolisthesis and failed back surgery syndrome.
Unfortunately, this study showed the TC group experienced more significant pain relief than the T group alone at 3 months this might have been from the effect of combined techniques peaking at 3 month, then it might have gradually worn off from steroid’s action, instability and the return of softened epidural adhesion and fibrosis15,22
However, Friedly JL et al.26 found epidural steroid injection plus lidocaine proposed insignificant benefit as compare with lidocaine separately in 400 patients who had lumbar central spinal stenosis and moderate to severe leg pain. On the other hand, they demonstrated interlaminar or transforaminal approaches which were not exactly same techniques as this study.
Recently, a retrospective study reported combined caudal and TFESI in herniated disc provided more significant pain relief and improved patient satisfaction than only TFESI at 1 year15. In contrast, this study showed no significant pain relief between the 2 groups from herniated disc in subgroup allocation, for which demographic data of our patients with herniated disc showed lower average ages (mean age 37 ± 8.5 and 35 ± 5.4 in the T and TC groups, respectively) than a related study (mean age 62.4 ± 15.5 and 57.6 ± 15.7 in the T and TC group, respectively) in which younger patients might have received greater benefit from steroid injection in accordance with Park et al.27 showing younger age produced a better response from TFESI. However, no significant difference was observed. Moreover, this study investigated just mild degree herniated disc such as mild unilateral paracentral disc herniation or mild foraminal stenosis and mild degree of spinal stenosis in which only the TFESI technique was sufficient to alleviate pain. Furthurmore, this study injected a larger volume in the T group (3.0 ml) compared with 1.5 ml in the study of TFESI by Kircelli A et al.15 in which a larger injected volume could cover more pain generators across multiple levels of the spine in accordance with Furman et al.25,28,29
In addition, this study postulated that synergistic anti-inflammatory effect from the double dose of steroid in the TC group may have conferred better pain relief. However, one related study reported 40 mg was as effective as 80 mg of methylprednisolone in TFESI for lumbar radicular pain29, while Kang et al.30 revealed no significant difference between 10, 20 and 40 mg of triamcinolone at 1 week in TFESI for disc herniation with lumbosacral radicular pain. Unsurprisingly, this study also showed no significant difference in herniated disc concerning different dosages of corticosteroid between the 2 subgroups analysis.
Our study had several limitations. Firstly, we demonstrated radicular pain from symptomatology and physical examination, for which the source of pain may have overlapped the pain referred pattern from the zygophysial joint, sacroiliac joint pain or enclosing soft tissues31–33, which might have limited the efficacy of the procedure. However, many common problems are involved in chronic low back pain. Secondly, electrodiagnosis was not performed in this study. Nonetheless, electrodiagnosis may have demonstrated false-negative findings, as demonstrated in a similar publication which showed 40 to 85% sensitivity depending on the referral range34,35. Thirdly, the study did not verify anterior or posterior epidural space of contrast flow which might have affected the efficacy of the result. Fourthly, this study included a small sample size for subgroup allocation which likely underpower analysis and might not have been able to detect differences between subgroups. A larger sample size in each etiology should be demonstrated in further study. Fifthly, this study provided two different approaches, which could be increase costs of procedures. Therefore, the higher volumes should be considerated if the effective result was occurred from higher volumes. Sixthly, some patients had bilateral radicular pain but only one side that more severe pain were provided in T group which might have effected for the result. Seventhly, this study did not collect exactly the duration of pain that can have huge effect on outcome as Bicket et al.36 ‘s propose. However, this study included only patients who had history of lumbosacral radicular pain more than 6 months. Lastly, we did not determine whether combination effect of oral medication, physiotherapy and placebo effect which might be effect for the result.
The additional effect of CESI to TFESI was more effective than TFESI separately at 3 months with no neurological deficit.
Figshare: data_epidural_04042020.xls. https://doi.org/10.6084/m9.figshare.12320846.v218
This project contains the following underlying data:
- data_epidural_04042020.xls (Pain and disability index data for study participants. Data dictionary provided as extended data36)
Figshare: Information of abbreviation data set. https://doi.org/10.6084/m9.figshare.12361499.v137
This project contains the following extended data:
Figshare: CONSORT checklist for ‘Effect of supraneural transforaminal epidural steroid injection combined with caudal epidural steroid injection with catheter in chronic radicular pain management: double blinded randomized controlled trial’ https://doi.org/10.6084/m9.figshare.1236149038
We acknowledge Dr. Nattaya Udomsukdi for visualizing and collecting data and Mrs. Pimrapat Tengtrakulcharoen for her statistical advice and data analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Anesthesiology and Pain management
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Anesthesiology, Pain management
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Datta R, Upadhyay K: A Randomized Clinical Trial of Three Different Steroid Agents for Treatment of Low Backache through the Caudal Route. Medical Journal Armed Forces India. 2011; 67 (1): 25-33 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Farrar J, Portenoy R, Berlin J, Kinman J, et al.: Defining the clinically important difference in pain outcome measures. Pain. 2000; 88 (3): 287-294 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: pain management
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Anesthesiology and Pain management
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pain Medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 24 Jul 20 |
read | read | read | |
|
Version 1 22 Jun 20 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)