Keywords
anti-IL-5 antibody, asthma control test, benralizumab, eosinophilic asthma, speed of onset, severe refractory asthma, asthma exacerbation
anti-IL-5 antibody, asthma control test, benralizumab, eosinophilic asthma, speed of onset, severe refractory asthma, asthma exacerbation
Based on the reviewers' requests, we now have added more information on the patient's clinical history and treatment adherence. Moreover, we discussed in detail the potential reasons for the unexpected rapid improvement in lung function after benralizumab administration and the novelty of our report compared to the previously published ones, as suggested by the reviewers.
See the authors' detailed response to the review by Paula Kauppi
See the authors' detailed response to the review by Claudio Micheletto
ACT, Asthma Control Test; BID, bis in die; FEV1, forced expiratory volume in 1 second, FVC, forced vital capacity; FeNO, fraction of exhaled nitric oxide; LAMA, long-acting muscarinic antagonist; IV, intravenous.
Acute exacerbation of severe asthma is a medical emergency that requires treatment with high-dose corticosteroids and accounts for >60% of the total costs of the disease care, primarily required for emergency visits and hospitalizations1,2.
Monoclonal antibodies are a relatively new therapeutic option for patients with severe refractory asthma, which can be used as an add-on to the maintenance therapy, with long-term effects such as reducing the need for systemic corticosteroids usage, improving asthma symptoms control and reducing exacerbations3. We report the use of a monoclonal antibody against interleukin-5, benralizumab, for the treatment of an acute exacerbation of severe asthma in a patient who refused to take systemic steroids.
We present the case of a 59-year-old non-smoker Caucasian female, lawyer, with a history of allergic asthma since childhood. She had no pets at home.
Timeline of patient's clinical evolution with diagnostic tests and treatments is shown in Figure 1.
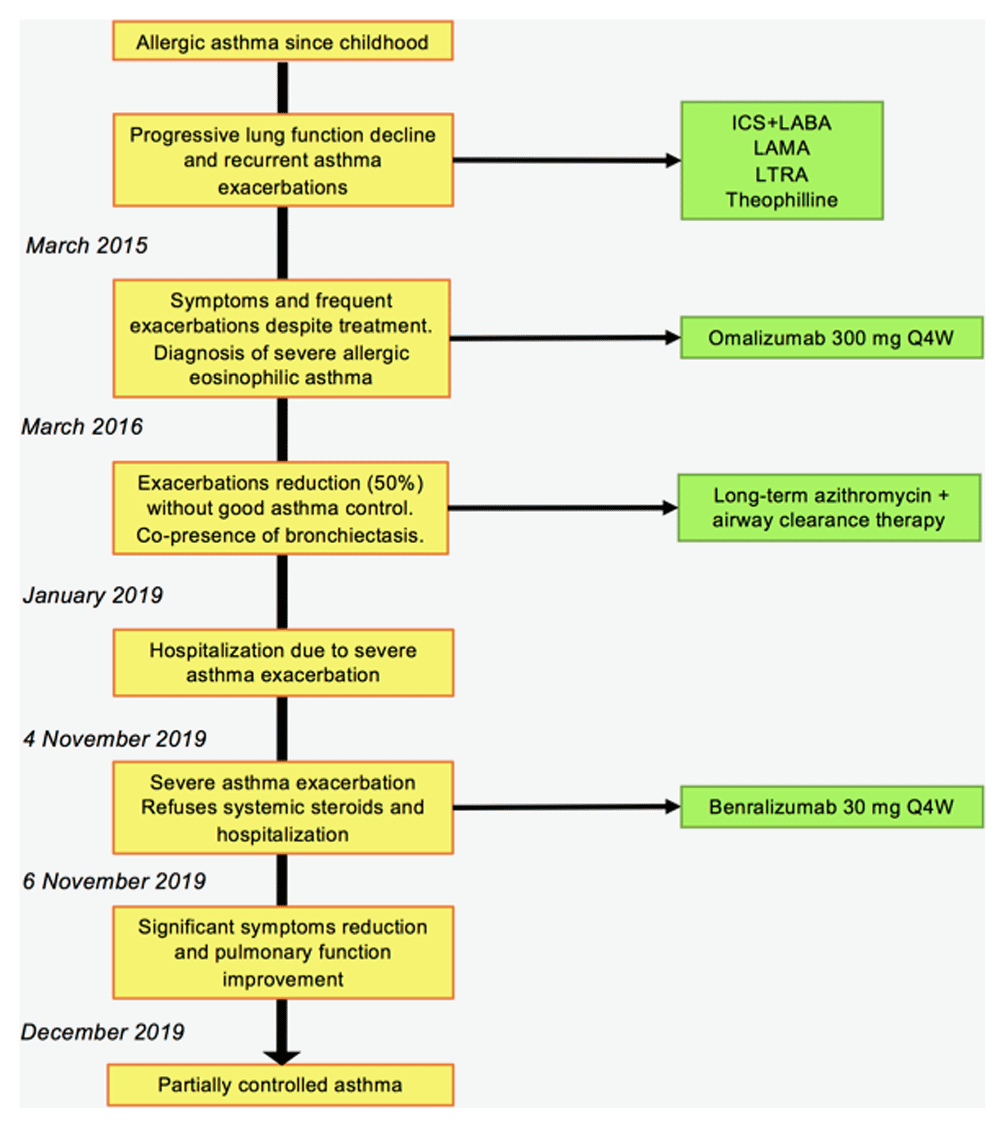
LAMA, long acting muscarinic agonist; LTRA, leukotrienes receptor antagonist; Q4W: every four weeks.
The patient had a body mass index within the normal range, chronic rhinosinusitis without nasal polyposis, and gastro-esophageal reflux under pharmacological control.
Her basal asthma regimen over the years was budesonide/formoterol 160 mcg/4.5 mcg, four puffs/day, with good treatment adherence and correct inhalation technique.
Over the past years, despite her adequate adherence to her maintenance regimen, the patient experienced a gradual worsening of her asthma symptoms, together with a progressive decline of her lung function and an increasing number of exacerbations, some of which required hospitalization. Therefore, after a pulmonologist consultation, combined treatment with a long-acting muscarinic antagonist (LAMA; 2.5 mcg tiotropium Respimat inhaler, two puffs daily), theophylline (300 mg tablets, bis in die) and a leukotriene receptor antagonist (montelukast, 10 mg daily) were added to her maintenance treatment regimen.
The patient’s adherence to both inhaled (ICS + LABA and LAMA) and oral (theophylline and montelukast) therapy continues to be very good, due to her fear of possible need for intravenous/oral steroids in case of non-adequate adherence.
In March 2015, she was referred to our outpatient respiratory clinic at Policlinico Vittorio-Emanuele di Catania, Italy, due to the persistence of asthma symptoms despite the therapy. Pulmonary function tests showed a forced vital capacity (FVC) of 78% of predicted value (2620 mL), and a forced expiratory volume in one second (FEV1) of 55% of predicted value (1400 mL), with a post-bronchodilator (after 400 mcg of salbutamol) increase in FEV1 of 24% (1990 mL).
She showed sensitization to multiple inhalant allergens by skin prick tests (house dust mites, dog and cat dander, and Parietaria judaica), high serum total IgE levels (201 IU/mL), high blood eosinophils count (670 cells/µL) and high fraction of exhaled nitric oxide (FeNO; 51 ppb). She complained of frequent exacerbations of her asthma symptoms and recurrent hospital admissions over the past year (nearly one/month) and, therefore, we classified her as severe refractory asthma according to Global Initiative for Asthma (GINA) guidelines4, after excluding other respiratory diseases that may share common clinical manifestations with severe asthma, as recommended by ERS/ATS guidelines5.
Moreover, the patient had always been reluctant to use systemic corticosteroids, referring to an almost immediate appearance of urinary retention, so she refused the proposed short course of oral corticosteroids. Therefore, we started treatment with omalizumab (300 mg via subcutaneous injections administered every four weeks).
We further evaluated our patient every four weeks, and she reported a dramatic reduction in the number and severity of exacerbations (nearly 50% less) and the number of hospitalizations overall, without using systemic steroids. Still, she described persistence of respiratory symptoms and poor asthma control with the need of a salbutamol inhaler at least five times a day, despite the maintenance and the biologic therapy. At her follow-up visit after one year from the first dose of omalizumab, her Asthma Control Test (ACT) score was 8, her FeNo was 33 ppb, and her pulmonary function tests showed an FEV1 of 73% of predicted value (1860 mL) and FVC of 90% of predicted value (3030 mL), an FEV1 / FVC of 61% and a positive post-bronchodilatation test response, with FEV1 reaching 85% (+ 12%) and 2105 mL. She underwent a chest computed tomography scan, revealing diffuse bronchiectasis; therefore, she started long-term azithromycin and airway clearance therapy on top of her usual maintenance treatment. The patient still achieved partially controlled asthma with treatment optimization.
In January 2019, she presented to the emergency department for a new severe exacerbation characterized by whistling dyspnea, greenish sputum, and acute respiratory failure, and required hospitalization.
During the hospital stay, her sputum cytological analysis showed 17% of eosinophils out of a total count of 250,000 cells/mL, and her blood tests exhibited an elevated peripheral eosinophilic count (470 cells/µL). Pulmonary function tests revealed an FEV1 of 49% of predicted, FVC of 72% of predicted and FEV1 / FVC of 57.4%. She started intravenous (IV) theophylline (240 mg intravenous slow bolus followed by a continuous infusion of 0.5 mg/kg/h), IV piperacillin/tazobactam (4.5 g every eight hours), IV corticosteroids (40 mg of methylprednisolone), and oxygen (nasal cannula 2 liters/minute) on top of her maintenance therapy, with progressive symptoms relieved. At discharge, we proposed a switch from omalizumab to mepolizumab, but the patient refused.
On November 4, 2019, she presented for a regular outpatient visit and complained of a worsening of her usual respiratory symptoms, presence of wheezing, reduced tolerance to physical activity, abundant purulent sputum, and frequent nocturnal awakenings. She had taken 25+ puffs of salbutamol to alleviate her chest tightness. Her oxygen saturation was 91% at rest.
Pulmonary function tests showed an FEV1 of 61% of predicted value (1480 mL), FVC of 74% of predicted value (2410 mL), and FEV1 / FVC of 61%. A post-bronchodilatation test FEV1 reached 70% (+9%) and 1700 mL. Her ACT score was 6. She also underwent blood tests, showing elevated eosinophils (390 cells/µL) and her FeNo was 60 ppb.
We proposed a short course of steroids, but she refused to take them due to the fear of urinary retention; she also refused hospitalization. Therefore, as a rescue solution, and due to the presence of elevated eosinophils, we decided to switch omalizumab to benralizumab (30 mg by subcutaneous injection every four weeks for the first three doses, and then every eight weeks thereafter) on top of her maintenance therapy. The same day the patient started the first administration of 30 mg subcutaneous injection of benralizumab.
At a telephone follow-up after 24 hours, she reported a significant improvement in her asthma symptoms with the almost total disappearance of sputum, absence of nocturnal awakening, and noticeable reduction of dyspnea, which made her stop rescue medication. At her 48 hours follow-up visit, the physical examination revealed a dramatic decrease in whistles as compared to the day before. The FEV1 was 80% of predicted value (1940 ml, +19% compared to two days before), FVC was 88% of predicted value (2890 ml, +14% compared to two days before) and FEV1 / FVC was 67% (+6%). Oxygen saturation has improved too, reaching 98% at rest, and her blood test showed complete depletion of eosinophils in peripheral blood. After four weeks, she returned to our outpatient service for administration of the second dose of benralizumab. She described good control of respiratory symptoms within the last month with no exacerbations, nocturnal awakening, nor sputum, and only occasional use of salbutamol. She did not report any adverse effects. Her blood count continued to show complete eosinophils depletion. Pulmonary function tests showed a further increase in lung function: an FEV1 of 98% of predicted value (2360 ml), FVC of 94% of predicted value (3140 ml), and FEV1 / FVC 75%. Her ACT reached a score of 18, her highest result ever and her FeNo was 47 ppb. Based on these excellent results the patients was then prescribed with benralizumab 30 mg every four weeks for the following two doses and then every 8 weeks, with the indication of performing a blood test to evaluate the blood eosinophils count before each biologic dose administration.
To the best of our knowledge, this case report is the first in the literature on the use of benralizumab administration during an acute attack of severe refractory eosinophilic asthma, without the concomitant use of systemic steroids. The main finding of this report is the efficacy and safety of benralizumab treatment during the acute phase of eosinophilic asthma exacerbation, showing a terrific and rapid response in terms of improvement of symptoms and pulmonary function (significant gain in FEV1 after only 48 hours), and reduction of sputum production, without the concomitant use of systemic corticosteroids and avoiding hospitalization.
Biologics have shown long-term beneficial effects in the management of severe asthma patients6. Characterizing the properties of one molecule versus another might be crucial for a more personalized treatment approach7. The speed of treatment onset might represent an essential underrated characteristic to consider in this context.
Omalizumab has been shown to reduce both asthma symptoms and exacerbations within the first 30 days of treatment8, while mepolizumab in months9 and reslizumab in weeks10. Indeed, benralizumab is responsible for rapid symptomatologic improvement, obtainable after only a few days11.
This brilliant and fast therapeutic effect is due to its antibody-dependent cell-mediated cytotoxicity activity, which results in a complete depletion of eosinophils in both peripheral blood and tissues12.
The rapid improvement in symptoms observed is in line with the results of a post-hoc analysis of two studies (SIROCCO and CALIMA), which showed positive impacts on symptoms by the third day after administration, reducing salbutamol use11. Our report strengthens the data from Nowak and coworkers13, who described similar rapid effects, indicating that the administration of one dose of benralizumab added to usual care in patients who presented with acute asthma to the emergency department decreased asthma exacerbation rate and severity as well as hospitalizations at twelve weeks.
A recent case report14 described a reduction in asthma symptoms after two days and an improvement in respiratory peak flow after four days of benralizumab administration14. Rapid response to treatment was previously described in another report from our group15.
The case presented is the first in which treatment with benralizumab during the acute phase of eosinophilic asthma exacerbation without the concomitant use of systemic steroids, on top of the maintenance treatment regimen, showed rapid resolution of symptoms within 24 hours. The immediate response to the treatment is also supported by a considerable increase in pulmonary function parameters (FEV1 +19%) obtained only after 48 hours, even without the concomitant use of systemic steroids. This case could be the starting point for the use of benralizumab in the acute phase of severe eosinophilic asthma exacerbations, starting the treatment early in the emergency room without resorting to systemic steroids. This rapid effect on lung function might be explained by the ability of benralizumab to reduce eosinophils not only in peripheral blood but also in the airways, limiting the release of noxious eosinophil granule proteins in bronchial tissue12,16.
Moreover, differently from previously published reports17,18 showing the rapid effects of biologics use together with high doses of systemic steroids and bronchodilators, our report is likely to reveal the real fast effect of benralizumab on acute asthma exacerbation, being used alone on top of the maintenance therapy, without concomitant use of systemic steroids.
This case report has some limitations. We did not report a long-term follow-up. Therefore, we cannot document long-term beneficial effects and treatment tolerance.
In conclusion, benralizumab may represent a feasible treatment as an add-on therapy in the management of acute asthma attacks, even without the use of systemic corticosteroids. Adequately powered multicenter trials are needed to confirm our observation.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of clinical details was obtained by the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: PK has received a fee for lecturing or for consultancy from TEVA, Novartis, GSK, Sanofi, AstraZeneca however, this has not affected my review
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Nowak RM, Parker JM, Silverman RA, Rowe BH, et al.: A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma.Am J Emerg Med. 2015; 33 (1): 14-20 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: severe asthma
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
References
1. Renner A, Marth K, Schäffl-Doweik L, Pohl W: Reslizumab in an invasively ventilated patient with acute respiratory failure. The Journal of Allergy and Clinical Immunology: In Practice. 2019; 7 (8): 2922-2923 Publisher Full TextCompeting Interests: PK has received a fee for lecturing or for consultancy from TEVA, Novartis, GSK, Sanofi, AstraZeneca however, this has not affected my review
Reviewer Expertise: Asthma, obstructive pulmonary disease, bronchiectasis, allergic diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 23 Jul 20 |
read | |
|
Version 1 23 Jun 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)