Keywords
mitochondria, arrhythmia, cardiac failure,Propofol
mitochondria, arrhythmia, cardiac failure,Propofol
We have added some sentences about how Propofol impairs carnitine palmitoyl transport activities and cardiac calcium dynamics, potentially affecting the oxidation of fatty acids. We have included a few sentences explaining the different findings between the past reports and the present study. In addition, we have made some minor changes to the text as suggested by the reviewers.
See the authors' detailed response to the review by Anja Karlstaedt
See the authors' detailed response to the review by Susanne Haen and Petra Fallier-Becker
PRIS, propofol infusion syndrome; 18F-FDG PET,18F-fluorodeoxyglucose positron emission tomography.
Propofol is extensively used in the intensive care units (ICU) for sedation1. Propofol infusion syndrome (PRIS) is widely recognized as an adverse event of this commonly used drug, but is rare and potentially lethal2. The pathophysiologic mechanism is still unknown. However, mitochondrial damage is suggested to be a potential pathogenesis mechanism. Here we report a severe case of PRIS with evidence of mitochondrial damage in both morphological and functional aspects.
A 22-year-old man, who was a healthy university student with Japanese ancestry without preexisting medical and family history, experienced muscle weakness and was admitted for the treatment of Guillain-Barré syndrome. On day six, he required mechanical ventilation due to progressive muscle weakness; propofol (3.5 mg/kg/hour) was administered via a peripheral venous catheter for five days for sedation. On day 13, he had hypotension with abnormal electrocardiogram findings (ST elevation in II, III, and aVF). Blood test revealed acute kidney injury, hyperkalemia and severe rhabdomyolysis (serum creatinine phosphokinase 271,700 IU/L, normal range 68-287 IU/L). He was transferred to our ICU on suspicion of PRIS by excluding other diagnoses. Administration of noradrenaline via a central venous catheter (0.3 µg/kg/min) and hemodialysis were initiated, and fasciotomy by orthopedic surgeons under general anesthesia without propofol was required for compartment syndrome of lower legs due to PRIS-rhabdomyolysis. Noradrenaline was gradually reduced and terminated on day 15. He gradually recovered from cardiac and renal dysfunction according to echocardiography and blood tests and was discharged from the ICU on day 30. On day 37, he repeatedly presented sinus bradycardia and right bundle branch block in continuous electrocardiogram monitoring, eventually requiring temporary pacing via the intracardiac placement of a pacing wire, with a finding of pericardial effusion on echocardiography. Detailed examination including cardiac 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) was conducted to evaluate whether these late-phase cardiac events were related to PRIS. Cardiac 18F-FDG PET on day 67 demonstrated heterogeneous 18F-FDG uptake in the left ventricle with a maximum Standardized Uptake Value(SUV) of 3.97 (Figure 1). Blood glucose level before imaging was 90mg/dL. Electron microscopic investigation of the endomyocardial biopsy, which was taken on day 75 to examine the cause of cardiac dysfunction, revealed abnormal findings in the mitochondria of the cardiomyocytes, including myelinization of the cristae (Figure 2), which was interpreted as mitochondrial damage. In addition, apoptotic myocytes were not observed. Since weakness of respiratory muscles and extremities muscles needed mechanical ventilation and rehabilitation, he was treated in the hospital for another 3 months.He was taken off the ventilator and transferred to another hospital on day 192 due to persisting muscle weakness, but with normal cardiac function without arrhythmia. Three-year follow-up revealed that he had normal cardiac function with normal activities of daily living.
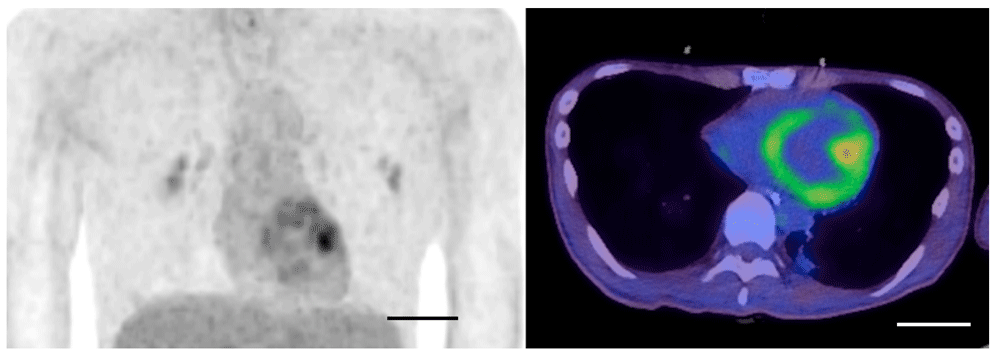
18F-fluorodeoxyglucose positron emission tomography showed heterogeneous 18F-FDG uptake in left ventricle. Scale bar: 5cm.
Mitochondrial damage is suggested as a potential pathogenesis of PRIS2–4. Mitochondrial damage was observed as a morphological finding in an electron microscopic evaluation of the heart in an autopsy case of PRIS5. We found myelinization of the cristae in cardiomyocyte on day 75; however, similar findings were not observed in postmortem electron microscopical image of mitochondria of PRIS in the previous report5. Different clinical course and timepoints may alter mitochondrial conditions. The autopsy study measured blood levels of short-chain acylcarnitines, while we have no blood sample available for the measurement. Further studies measuring blood levels of short-chain acylcarnitines would strengthen the case results. Similarly, mitochondrial damage was observed in the endomyocardial biopsy two months after the onset in the present case. Mitochondrial damage can also be detected as a functional impairment of fatty acid utilization with alternatively increased glucose utilization6. Propofol is known to inhibit the effects of carnitine palmityl transferase 1 (CPT 1), which transports long-chain fatty acids into the mitochondria3. Thus, propofol potentially impairs carnitine palmitoyl transport activities and cardiac calcium dynamics, affecting the oxidation of fatty acids3. The uptake of a glucose analog (18F-FDG) in left ventricle on day 67 (Figure 1) in the present case implies a shift in the energy substrate of cardiomyocytes from fatty acid to glucose, suggesting mitochondrial damage. To the best of our knowledge, this is the first to report a case of PRIS with evidence of mitochondrial damage in both morphological and functional aspects, which is the strength of this case report. The evidence of increased glucose uptake by 18F-FDG PET and mitochondrial damage by electron microscopic investigation was not repeatedly evaluated during the time-course but a single time-point (18F-FDG PET on day 67 and endomyocardial biopsy 75), which is a potential limitation. Additional timepoints data in future studies would reveal that these flux changes occur due to mitochondrial damage or pharmacologic modulation of key regulatory enzymes and transporters. Since the mitochondrial damage was detected 2 month later after PRIS onset, sustained mitochondrial damage may be a therapeutic target beyond the initial therapy of discontinuing propofol administration.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiology, Metabolism, Systems Biology, Computational modeling.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pathology, Neuroscience, Electron Microscopy
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
References
1. Karlstaedt A, Zhang X, Vitrac H, Harmancey R, et al.: Oncometabolite d-2-hydroxyglutarate impairs α-ketoglutarate dehydrogenase and contractile function in rodent heart. Proceedings of the National Academy of Sciences. 2016; 113 (37): 10436-10441 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiology, Metabolism, Systems Biology, Computational modeling.
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pathology, Neuroscience, Electron Microscopy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 20 Jan 22 |
read | read |
|
Version 1 16 Jul 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)