Keywords
karyotype, chromosome, centromere, histone H3, Oikopleura, oocyte, embryo, H3S28P
karyotype, chromosome, centromere, histone H3, Oikopleura, oocyte, embryo, H3S28P
Karyotyping is a long-established histochemical method to visualize chromosomes of eukaryotes (Hsu & Benirschke, 1967; Tjio & Levan, 1950). A multi-dye reagent developed at the turn of the 20th century for the diagnosis of infections in human histological preparations (Giemsa, 1902; Giemsa, 1904) was later used to stain chromosomes themselves in order to study their numbers, translocations, and other aberrations. This rapid technique, involving the use of stains including methylene blue, eosin, and azure B allows for observation of chromosomes with a simple light microscope, naturally lending itself to a first attempt for karyotyping analysis.
Although individual chromosomes have been resolved by histochemical techniques in O. dioica, the reported results differ in numbers from n=3 (Körner, 1952) to n=8 (Colombera & Fernaux, 1973). More recently, metaphase-specific histone 3 (H3) markers have been used to determine the structure and the segregation of genetic material during oogenesis in situ (Ganot et al., 2006; Schulmeister et al., 2007) while providing greater detail and resolution. One such marker is histone H3 phosphorylated at Ser-28 (Kawajiri et al., 2003); although it is typically used to identify centromeres during metaphase (Kurihara et al., 2006), we observed in data presented in previous studies that signals were not confined to centromeres. More importantly, the localization of the H3S28P signal depends on the phase of the cell cycle: spatially punctate signals were found evenly spread within the nuclear envelope during prophase, while condensed chromatin gave an outlined staining of the sister chromatids during metaphase in a manner consistent with alignment along the metaphase plate (Table 1; Campsteijn et al., 2012; Feng & Thompson, 2018; Feng et al., 2019; Olsen et al., 2018). Moreover, a structure in which genetic material is sequestered in a ∏-shaped conformation has been observed during meiotic cell divisions between the final phases of oogenesis and mature oocytes (Ganot et al., 2008). However, these results were all obtained from the same laboratory strain originating from the Atlantic Ocean. Considering the discrepancy of past findings, and the fact that our laboratory strain originates from a geographically distinct ocean, we applied H3S28P staining on intact embryos and oocytes to confirm the chromosome count and validate our genome sequencing assemblies of Okinawan O. dioica marine populations among the Ryukyu Islands of southern Japan.
Histochemical staining. Live specimens were collected from Ishikawa Harbor (26 °25'39.3 "N, 127 °49'56.6 "E) by a hand-held plankton net and cultured in the lab (Masunaga et al., 2020). Mature females were collected prior to spawning, individually washed with filtered autoclaved seawater (FASW) 3 times for 10 minutes and placed in separate 1.5 ml tubes containing 500 µl of FASW. Nearly mature males, full of sperm, were also washed 3 times in FASW. Mature males that successfully made it through the washes intact were placed in 100 µl of fresh FASW and allowed to spawn naturally. As soon as females spawned, each individual clutch of 100-200 eggs was washed three times for 10 minutes by moving eggs along with a pulled capillary micropipette from well to well in a 6-well dish, each containing 5 ml of FASW, and left in a fresh well of 5 ml FASW in the same dish. These were stored at 17 °C and set aside for fertilization. Staged embryos were initiated by gently mixing 10 µl of the spawned male sperm with the awaiting eggs in FASW at 23 °C. Developing embryos were staged and collected by observation under a Leica M165C dissecting microscope. These embryos were quickly dechorionated using 0.1% sodium thioglycolate and 0.01% actinase in FASW for 2–3 minutes, then promptly washed with 2 washes with filtered autoclaved seawater prior to fixation and staining. Unfertilized eggs were treated similarly.
Embryos were Giemsa stained as previously described in Shoguchi et al., 2005. Briefly, approximately 20–30 dechorionated embryos were treated with 0.04% colchicine in FASW for 30 minutes and then treated with decreasing amounts of KCl (50 mM and 25 mM) for five minutes each. Fixation was quickly performed with cold methanol:glacial acetic acid (3:1). The fixative was changed three times in the span of 18 hours while at -30 °C. The next morning, the fixed cells were quickly resuspended in 60% Acetic acid and methodically dropped from a height of 7 – 8cm onto a 48°C pre-warmed slide (Matsunami Glass, S2441). The slide was incubated for an additional 2 hours at 48°C; then stained with 6% Gimesa in 67mM sodium phosphate pH 7.0 for 2 hours at room temperature and rinsed with ddH2O. These were dried for two hours at room temperature, mounted with DPX Mountant (Sigma, 06522) and covered with No.1 35 x 50 mm glass coverslips (Matsunami Glass, C035551).
Immunostaining. Washed eggs and embryos were immediately fixed in 4% w/v paraformaldehyde, 100 mM MOPS pH 7.5, 0.5 M NaCl, 0.1% triton-X100 at 23 °C ON (Campsteijn et al., 2012). The samples were then washed for 10 minutes once with PBSTE (PBS supplemented with 1 mM EDTA) and 3 times for 10 min with PBSTEG (PBS supplemented with 1 mM EDTA and 0.1 M glycine). The samples were blocked using PBSTE supplemented with 3% bovine serum albumin at 4 °C overnight. Rabbit polyclonal (Thermo Fisher Scientific Cat# 720099, RRID:AB_2532807) or rat monoclonal (Abcam Cat# ab10543, RRID:AB_2295065) primaries directed against H3S28P were diluted 1:100 in PBSTE 3% BSA and incubated at 4 °C for 3 days. The next morning, these were washed in PBSTE for 10 minutes 3 times and incubated with anti-rabbit (Thermo Fisher Scientific Cat# A-11034, RRID:AB_2576217) or anti-rat (Molecular Probes Cat# A-11006, RRID:AB_141373) Alexa488 conjugated secondary antibodies diluted 1:500 with PBSTE 3% BSA at 4 °C ON. The following morning, samples were washed 3 times for 10 min with PBSTE. The samples were mounted on cleaned glass slides (Matsunami Glass, S2441) with fluorescence preserving mounting medium (ProLong. Fluoromount G Mounting Medium, RRID:SCR_015961) covered with No.1 35 x 50 mm glass coverslips (Matsunami Glass, C035551) and sealed with nail polish.
Both a Nikon Ni-E epifluorescent and a Zeiss LSM 510 Meta confocal microscopes were used to acquire Z-stack images of eggs and embryos. Brightfield images were obtained using a 20x/0.75 CFI Plan Apo λ objective (Nikon, MRD00205) for histochemical staining. Epifluorescent immunofluorescent images were obtained with both 20x/0.75 and 40x/0.95 CFI Plan Apo λ air objectives (Nikon, MRD00405); each sample acquisition was Z-stacked with each plane set at an interval of 1 µm. Confocal images were acquired using a 40x/0.75 EC Plan-Neofluar M27 (Zeiss, 420360-9900-000) and 63x/1.4 Plan-Apochromat M27 oil immersion (Zeiss, 420782-9900-79) objectives; each sample acquisition was Z-stacked, line averaged twice with each plane set at an interval of 0.6 and 0.27 µm, respectively.
Images acquired from a Nikon Ni-E epifluorescent were deconvoluted with Nikon Elements-AR v5.0 software. Images for both epifluorescent and confocal acquisitions were analyzed using Imaris software SPOT DETECTION tool (Imaris, RRID:SCR_007370) for embryos and unfertilized eggs, parameters set at 0.5 and 0.43 µm spot detection size, respectively, and software preset to QUALITY auto signal threshold for each individual cell within a sample. Alternatively, ImageJ v1.51 3D Objects Counter may be employed to count signals. Epifluorescent and confocal acquisitions of embryos and their subsequent analysis were performed independently by different researchers to exclude bias.
Initial attempts at visualizing individual chromosomes were done with developing embryos and Giemsa staining. The spreads from two time points, 32- and 64-cell developmental stages, gave results with counts ranging between 11–27 stains per cell (BioImage Archive, S-BIAD21, Experiment A). Although hypotonic-induced cell spreads were confined as a result of incomplete dechorionation and digestion with the enzymatic dissociation cocktail, groups of chromosomes were easily associated to a single cell. However, individual chromosomes were difficult to resolve due to the low resolution of images. In order to eliminate possible miscounts and other Giemsa staining artifacts, immunostaining was used to count individual chromosomes using a centromere-specific primary antibody directed against H3S28P and a secondary antibody conjugated to Alexa488 directed against the primary antibody.
Signal-based thresholding was employed to determine the number of distinct 515 nm emission signals present in acquired images from epifluorescent and laser confocal microscopes (BioImage Archive, S-BIAD21, Experiment B & D). The data was analyzed using the Imaris SPOT DETECTION tool (Oxford Instruments). Two types of nuclei were apparent within each embryo: nuclei containing evenly distributed, clearly separated spots that were interpreted as being in prophase (Figure 1A and 1B, blue circles) and nuclei with intense clusters of signals in the center, considered to be in metaphase (Figure 1A and 1B, red squares). Counts from these two classes of nuclei fall into separate distributions (Figure 1C and 1D). Both epifluorescent and confocal acquisitions were in near agreement, epifluorescence n = 20, mean 6.2 , 95% CI 5.6 – 6.8; confocal n = 13, mean 6.4, 95% CI 5.7 – 7.1 and epifluorescence n = 20, mean 12, 95% CI 11.0 – 13.0; confocal, n = 14, mean 14.1, 95% CI 12.9 – 15.3. We interpret the results as a count of 12 distinct centromeres in prophase cells and a count of 6 larger spots identifying pairs of centromeres in metaphase (Figure 1B).
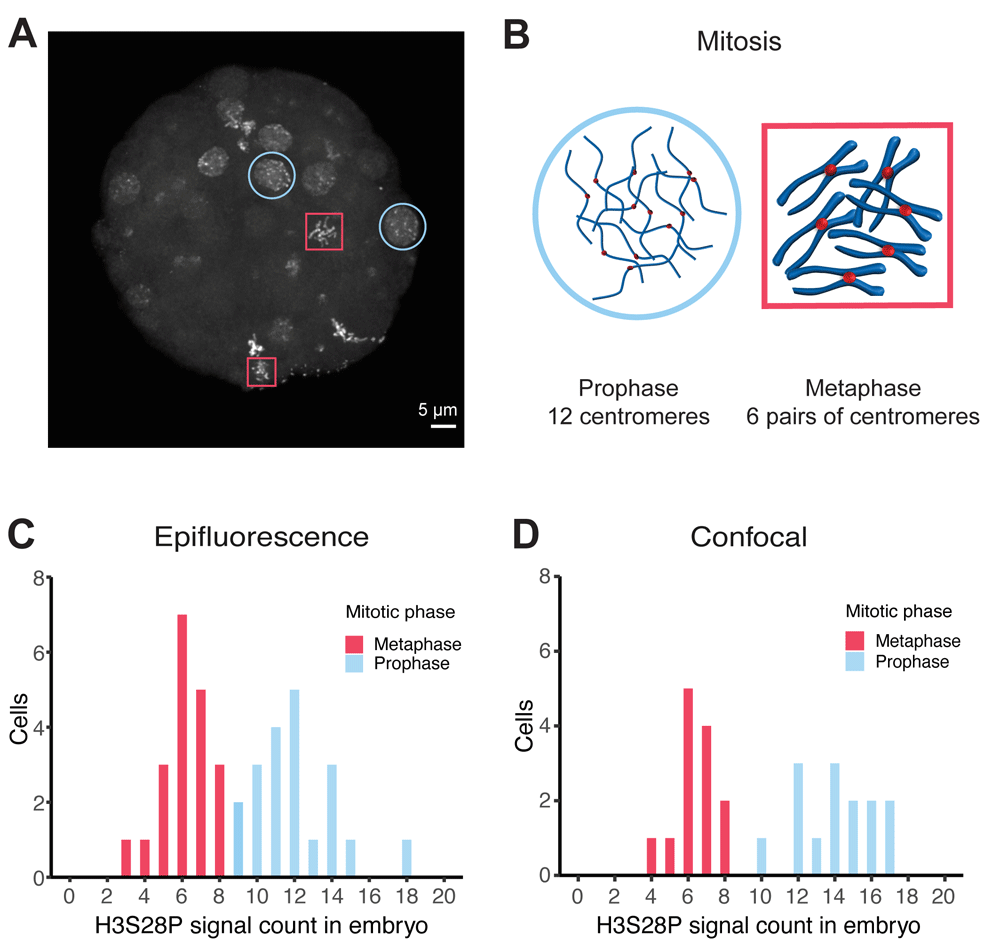
Anti-H3S28P rabbit-derived polyclonal stained 64-cell whole-embryo chromosomal imaging data analyzed by Imaris software SPOT DETECTION tool using different microscopy techniques. A Maximum projection of confocal image of an embryo demonstrating the differences in signal localization appearance and signal count, which was inferred to represent distinct cell cycle phases. Red box, metaphase; blue circle, prophase. B Schematic interpretation of signals with respect to chromatin structure during prophase and metaphase cell cycle states. As a simplification, all chromosomes have been drawn at an equal length although they actually vary in O. dioica. C Distribution of signal counts within individual cells using epifluorescent (n = 40) and D confocal (n = 27) microscopes. Two distinct populations were observed in a bimodal distribution, which corresponded with cell cycle stage. Red, metaphase; blue, prophase.
To confirm our observations on germ cells and therefore rule out polyploidy, which is frequent in O. dioica’s somatic cells (Ganot & Thompson, 2002), we also analyzed oocytes in prometaphase I before fertilization (Schulmeister et al., 2007). We identified confined groupings of signals in unfertilized eggs (Figure 2A; BioImage Archive, S-BIAD21, Experiment E). Images were analyzed using the Imaris SPOT DETECTION tool to determine chromosome counts and their distributions (Figure 2B). Counts from the compact rosette-shaped genetic material averaged near 6 (n = 23, mean 5.70, 95% CI 5.2 – 6.2). Visual inspection of individual Z-sections (Figure 2C) confirm agreement with the Imaris count analysis and annotation (Figure 2D). We interpret these results as each spot corresponding to a pair of centromeres from paired chromatids forming a synapsis in unfertilized eggs (Figure 2E).
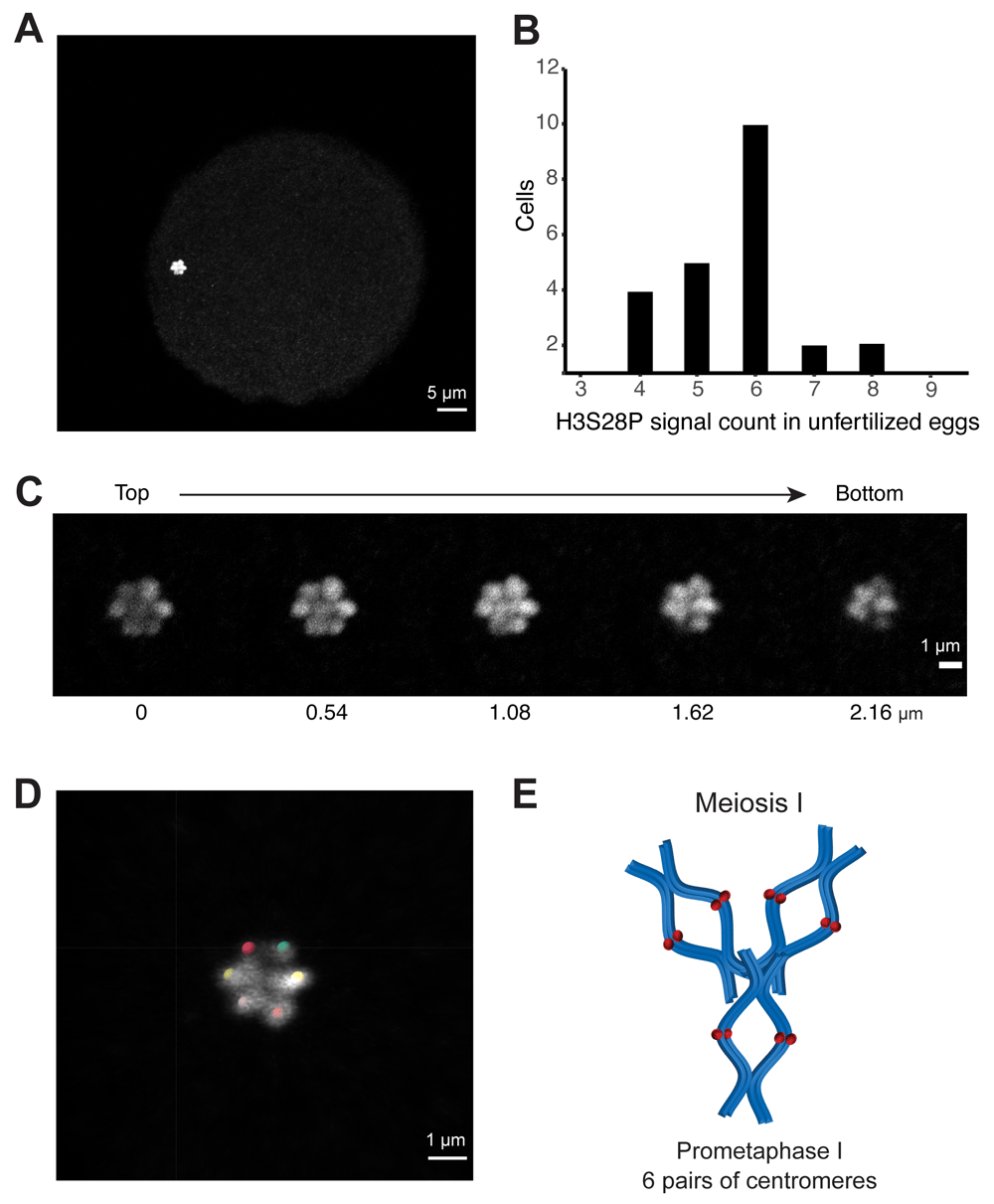
A Maximum signal projection of a representative confocal Z-stack acquisition of anti-H3S28P rat monoclonal stained oocyte used for the count analysis. B Distribution of signal counts from centromere-stained oocyte genetic material, analyzed by Imaris software SPOT DETECTION tool (n = 23). C Individual Z-sections from same image acquisition showing the 3D structure of the genetic material, each plane is 0.54 µm apart. D Imaris spot analysis and annotation of signal positions from Z-stack acquisition. E Schematic representation of our interpretation that each signal is a pair of closely associated centromeres from a pair of sister chromatids. As a simplification, all chromosomes have been drawn at an equal length although they actually vary in O. dioica.
Despite the variation in signal counts across different image acquisitions settings, a haploid chromosomal count of three provides the most parsimonious explanation of the collected data and agrees with previously published assemblies (Denoeud et al., 2010).
Oocyte staining with rat anti-H3S28P and a conjugated secondary fluorophore gave rise to a compact area in which signals appear to stack on top of one another (Figure 2A). Previously, DNA stains at this stage have been interpreted as a structure resembling the Greek character ∏ (Ganot et al., 2007), representing condensed chromosomes seen in mature oocytes arrested in meiosis I. Our data does not include DNA stains and therefore our illustration (Figure 2E) should not be interpreted as precluding the previously reported ∏-structure.
Currently, the sequence of the centromeres is not known, although chromatin immunoprecipitation with a H3S28P antibody followed by long-read sequencing might be able to provide this information. However, our whole embryo staining data and the previous literature (Table 1) show that non-centromeric signal present outside metaphase stages may introduce noise. Thus, alternative targets such as other centromeric histone 3 variants (Moosmann et al., 2011) might be preferable. Availability of centromeric sequences would open the possibility of confirming our results with fluorescence in situ hybridization.
In summary, we conclude that the Okinawan Oikopleura dioica genome consists of three pairs of chromosomes in diploid cells. We believe that the images may be useful for examining cell cycle specific changes to chromosome structure and encourage the reuse and reanalysis of our data located in the EBI BioImage Archive (Ellenberg et al., 2018).
Image acquisitions: Image data are available in the BioImage Archive
Centromere specific antibody mediated karyotyping of Okinawan Oikopleura dioica. Accession number S-BIAD21. https://www.ebi.ac.uk/biostudies/preview/studies/S-BIAD21
We thank Drs. Daniel Chourrout, Hiroki Nishida & Eiichi Shoguchi for discussions and suggestions regarding the subject matter. Additionally, great appreciation is given the staff (Drs. Toshiaki Mochizuki, Shinya Komoro & Paolo Barzaghi) in the Imaging Section of the Research Support Division at OIST for providing technical assistance. Finally, we are grateful for Dr. Michael Mansfield and Charlotte West comments on the manuscript’s draft and appearance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tunicate embryogenesis, asexual reproduction, and evolutionary developmental biology. Transcriptional regulation.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: cell cycle, oogenesis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 01 Mar 21 |
read | read |
|
Version 1 28 Jul 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)