Keywords
lentiform fork sign, basal ganglia lesion, diabetic uremic syndrome, metformin, consciousness disturbance
lentiform fork sign, basal ganglia lesion, diabetic uremic syndrome, metformin, consciousness disturbance
We responded to each reviewer’s comments as follows.
1) In the part of Case report, we added the extra information about hemodialysis conditions: quantity of blood flow of 200 mL/min, quantity of dialysate flow of 500 mL/min, 1.5 m2 -dialysis membrane made of polymer alloy (polyarylate/polyethersulfone). Furthermore, we mentioned the time interval of hemodialysis treatment after recovery.
2) In the amendments from version 1 of the PDF file of this article, we described that the level of metabolic(lactic) acidosis on admission was also much mild not so high. As written here, we modified “severe lactic acidosis was not observed” to “the level of metabolic acidosis on admission was not very high (bicarbonate, 18.1 mEq/L; lactic acid, 6.2 mmol/L)” in the part of Discussion.
See the authors' detailed response to the review by Ping-Hsun Wu
See the authors' detailed response to the review by Shashwati Sarkar
Metabolic encephalopathy with abnormal basal ganglia lesions has been reported in hemodialysis patients. Ingestion of some types of mushroom, star fruit, and drugs (e.g., anti-herpes virus drugs) can cause encephalopathy in these patients1–3. In particular, diabetic dialyzed patients can present with bilateral symmetrical low densities in the basal ganglia on brain computed tomography (CT), with a bilateral symmetrical hyperintensity in the same area and a lentiform fork sign on T2-weighted MRI4–10. In addition to diabetic uremic syndrome (DUS)4,5, the lentiform fork sign can be observed in severe metabolic acidosis11–13, dialysis disequilibrium syndrome14, and metformin-associated encephalopathy (ME)6,7. The pathogenic basis of this sign is considered to relate to cytotoxic edema based on the severity of metabolic acidosis8,11. Intensive dialysis is a therapeutic option for removing the uremic toxins, to correct metabolic acidosis and remove medications. Herein, we present a case of a 57-year-old Japanese man in whom the lentiform fork sign was a clue for the differential diagnosis of ME or DUS. Metformin tends to increase lactate production and result in metabolic acidosis in ME6,7,9,10, while chronic hyperglycemia with coexistence of uremic toxins and metabolic acidosis is the main mechanism in DUS4,5. Which of these is the main cause in our case presenting with the lentiform fork sign is discussed below.
A 57-year-old Japanese man who had been on maintenance hemodialysis three-times weekly for four years because of diabetic nephropathy developed gait and consciousness disturbance (the Glasgow Coma Scale score of E3V4M6), fatigue, numbness in his left upper limb, and a slow response during conversation approximately 10 days before admission. His wife denied him taking mushrooms or star fruit, which can cause consciousness disturbance in hemodialysis patients. There were no abnormal neurologic findings on physical examination. However, bilateral symmetrical basal ganglia lesions were noted on brain CT (Figure 1a).

a, e Head computed tomography (CT) and b–d head MRI (T2-weighted image). a, b High-resolution lesions in the bilateral symmetrical basal ganglia were evident at admission. c, d The bilateral symmetrical basal ganglia lesions gradually improved on the 18th hospital day and at three-month follow-up. e However, the basal ganglia lesions remained at eight-month follow-up.
On admission to our hospital, his consciousness was disturbed, such as he only could open his eyes following calling, and he had difficulty sitting alone. He showed a tonic planter reflex on physical examination. His blood pressure was 190/91 mmHg, and his heart rate was 104 beats per min. Arterial blood gas analysis showed a pH of 7.37, bicarbonate ion of 18.1 mEq/L, and lactic acid of 6.2 mmol/L (normal, 0.5–1.6 mmol/L). Serum vitamin B1 (thiamin) level was 45 ng/mL (normal, 24–66 ng/mL). Serum vitamin B1 (thiamin) level was 45 ng/mL (normal, 24–66 ng/mL). Serum calcium and blood aluminum levels were all within the acceptable range. Kidney function data sampled the day after dialysis, blood urea nitrogen, and serum creatinine were consistent with dialysis. His HbA1c was 5.8% on admission.
Brain MRI showed bilateral symmetrical basal ganglia lesions with an expansile high signal intensity (lentiform fork sign) on T2-weighted sequences (Figure 1b), which was not seen on MRI taken one-year prior when he developed a right thalamic lacunar infarction.
In his medication history, he had taken metformin for six months. His wife said that his sluggish speaking and dysarthria appeared gradually after starting metformin treatment (Figure 2). His plasma metformin concentration was extremely high (25,700 ng/mL). Thus, we considered that metformin may have initially caused the encephalopathy. However, we also considered the possibility of DUS, because his gait and consciousness disturbance appeared relatively rapidly approximately 10 days before hospitalization. DUS typically occurs in uncontrolled uremic patients with diabetic mellitus.
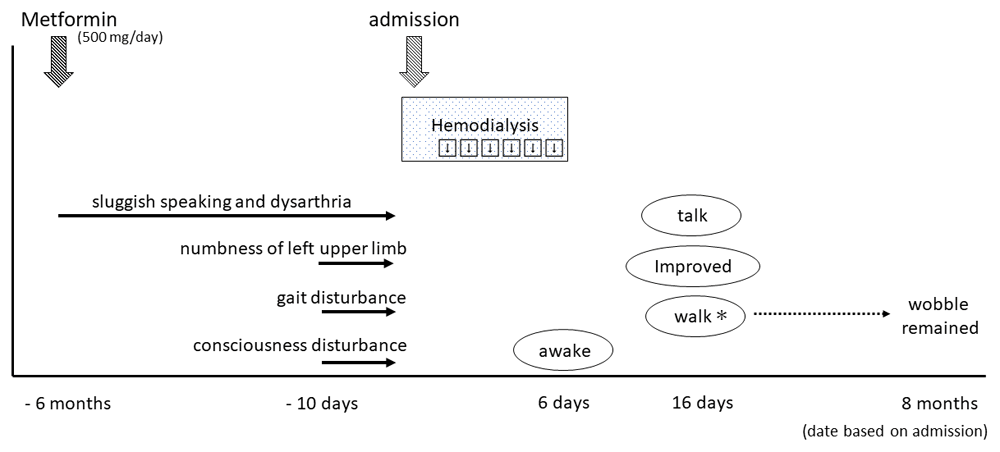
Since that time, he developed gradual symptoms of sluggish speaking and dysarthria, while numbness of his left upper limb, gait disturbance, and consciousness disturbance appeared 10 days before admission. He received emergency consecutive hemodialysis for six days, after which he awoke, and was gradually able to walk and talk. *Walking was possible, but wobbling during walking remained at eight-month follow-up.
In either case, we stopped metformin treatment, and immediately performed intensive hemodialysis (four hours daily; blood flow, 200 mL/min; dialysate flow, 500 mL/min; 1.5 m2-dialysis membrane, polymer alloy (polyarylate/polyethersulfone) for six days after hospitalization to remove metformin and uremic toxin, and to correct metabolic acidosis. The first dialysis session reduced his lactic acid levels from 6.2 to 1.3 mmol/L. After six consecutive sessions of hemodialysis, his consciousness was restored, and his tonic plantar reflex disappeared. Subsequently, we performed hemodialysis treatment for four hours per day, three times a week on the same hemodialysis conditions as above. After starting meals, linagliptin was chosen as an anti-diabetic drug to replace metformin.
On the 18th hospital day, T2-weighted brain MRI revealed a modest improvement in the lentiform fork sign (Figure 1c). The patient was gradually able to sitting alone, walk, and talk with staff and his wife. He was discharged from our hospital within one month.
At three-month follow-up, the lentiform fork sign was further improved on brain MRI (Figure 1d). However, at eight months after the onset, he still complained movement disorders, such as a wobble when walking and body tilting when resting. Brain lesions were still evident on CT scan (Figure 1e).
Herein, we report a diabetic hemodialysis patient with consciousness disturbance who presented with the lentiform fork sign on T2-weighted brain MRI. This finding appears in the basal ganglia, which is vulnerable to addictive toxins and metabolic products8,12. The lentiform fork sign is comprised of the following elements: 1) the lateral arm, formed by the edematous external capsule and extending from the anterior end of the putamen to the stem; 2) the stem, created by merging of the edematous external and internal capsules at the inferoposterior end of the putamen; and 3) the medial arm, which extends from the stem anteriorly up to one third of the medial edge, where it splits into two slightly less T2/FLAIR-hyperintense branches engulfing the globus pallidus11,12,15. In the present case, brain MRI showed the same expansile high signal intensity (Figure 1b). The lentiform fork sign is rare but non-specific. Thus, a differential diagnosis should be considered (Table 1)8,11–15, of which ME or DUS may be the cause in the present case.
| a) Uremic encephalopathy * |
| b) Severe metabolic acidosis |
| c) Ketoacidosis |
| d) Dialysis disequilibrium syndrome |
| e) Intoxication (methanol, ethylene glycol, etc) |
| f) Drug-induced (metformin) |
The use of metformin in dialyzed patients can cause drug accumulation in the brain, leading to neurological abnormalities, difficulties of speech and walking, with worsening of sensory disturbance, tiredness, drowsiness, and weakness (i.e., ME)6,7,9,10. Metformin is first-line drug used in type 2 diabetes mellitus. However, it is contraindicated in patients with an estimated glomerular filtration rate <30 mL/min/1.73 m2, because of an increased risk of lactic acidosis. Acidosis can damage the basal ganglia, resulting in cytotoxic edema7, which is sometimes irreversible despite intensive hemodialysis to remove metformin and lactic acid, and to correct acidosis. According to the previous reports, hemodialysis patients are at risk for thiamin deficiency which is induced to encephalopathy, because they are in the condition of malnutrition and tend to lose water-soluble vitamins in the hemodialysis procedure16,17. Furthermore, thiamin deficiency may be a possible mechanism in metformin-induced encephalopathy18. In our case, thiamin level was not decreased and the level of metabolic acidosis on admission was not very high (bicarbonate ion, 18.1 mEq/L; lactic acid, 6.2 mmol/L). In addition to these, the occurrence of gait disturbance, severe dysarthria, and consciousness disturbance was subacute even though sluggish speaking and dysarthria had been gradually worsening six months before starting taking metformin as shown in Figure 2, and the patient was neither malnutrition nor weight loss. That is why it was not necessarily ME.
Alternatively, DUS is characterized by acute or subacute progression with a variety of movement disorders such as gait disorders, dysarthria, parkinsonism, and consciousness disturbance. DUS can cause bilateral symmetrical basal ganglia lesions on brain CT and T2-weighted MRI4,5 in patients with diabetic nephropathy, even if they are not on hemodialysis. To date, approximately 30 cases of DUS have been reported, many of which are Asian. The reported risk factors of DUS include a high level of HbA1c before and at hemodialysis, and increasing metabolic acidosis. Hyperglycemia damages the microvasculature, resulting in a fragile vascular smooth muscle, and the accumulation of uremic toxins and/or metabolic acidosis can damage the blood-brain-barrier, leading to altered metabolism and homeostasis in the brain. This can result in basal ganglia injury, including angiogenic edema, which is reversible and shows favorable prognosis.
The clinical presentation in our case was not helpful for differentiating ME and DUS, because these symptoms were indistinguishable (Table 2). Initial hemodialysis improved lactic acidosis, although intensive hemodialysis for six consecutive days was required to improve his consciousness. The lentiform fork sign on MRI improved at first, although brain CT findings at eight-month follow-up showed low density signals in those regions, and his neurological sequelae remained, suggestive of continued cytotoxic edema. ME was likely the main cause of injury in our case. Nevertheless, the patient’s condition worsened relatively rapidly before admission, similar to that seen in DUS. DUS can also contribute to cytotoxic edema in the basal ganglia, and has a variable progression. Thus, DUS may have also contributed to the encephalopathy in our case.
In summary, we report a diabetic hemodialysis patient with encephalopathy presenting as the lentiform fork sign derived from ME and/or DUS. In dialysis patients showing gait and consciousness disturbance, the lentiform fork sign on brain CT and T2-weighted MRI may be useful for differential diagnosis.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
We thank Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical nephrology, hemodialysis, chronic kidney disease
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endocrine disorders, Assisted reproductive technology, Infertility, Gynaecology, Obstetrics
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. McGarvey C, Franconi C, Prentice D, Bynevelt M: Metformin-induced encephalopathy: the role of thiamine.Intern Med J. 48 (2): 194-197 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Endocrine disorders, Assisted reproductive technology, Infertility, Gynaecology, Obstetrics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 02 Dec 21 |
||
|
Version 2 (revision) 20 Oct 21 |
read | read |
|
Version 1 11 Aug 20 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)